Advertisement

Microplastics in aquatic systems: A comprehensive review of its distribution, environmental interactions, and health risks
- Research Article
- Open access
- Published: 13 December 2024
Cite this article
You have full access to this open access article

- Divya Pal ORCID: orcid.org/0000-0001-7027-147X 1 , 2 ,
- Roshan Prabhakar 3 ,
- Visva Bharati Barua 4 ,
- Ivar Zekker 5 ,
- Juris Burlakovs 6 ,
- Andrejs Krauklis 7 ,
- William Hogland 8 &
- Zane Vincevica-Gaile 9
927 Accesses
1 Altmetric
Explore all metrics
Microplastics (MPs) have become a critical pollutant, accumulating in aquatic ecosystems and posing significant environmental and human health risks. Approximately 5.25 trillion plastic particles float in global oceans, releasing up to 23,600 metric tonnes of dissolved organic carbon annually, which disrupts microbial dynamics. MPs arise from the breakdown of larger plastics, degraded by photodegradation, thermal degradation, and biological processes, which are influenced by polymer type and environmental factors. As carriers, MPs absorb and transport contaminants such as heavy metals, per- and polyfluoroalkyl substances (PFAS), and persistent organic pollutants (POPs) across trophic levels, thereby increasing toxicity within food webs. Key aquatic organisms, including microalgae, molluscs, and fish, experience cellular toxicity, oxidative stress, and disruptions in essential functions due to MP ingestion or adhesion, raising concerns about their bioaccumulation in humans through ingestion, inhalation, and dermal contact. The complex surface chemistry of MPs enhances their pollutant adsorption, a process modulated by environmental pH, salinity, and contamination levels, while aging and structural attributes further impact their bioavailability and toxicity. This review consolidates knowledge on MPs’ occurrence, transformation, pollutant interactions, and methodologies for sampling and analysis, emphasizing advancements in spectroscopy and imaging techniques to improve MP detection in aquatic environments. These insights underscore the pressing need for standardized analytical protocols and comprehensive toxicological research to fully understand MPs’ effects on ecosystems and human health, informing future mitigation strategies and policy development.
Graphical abstract
Similar content being viewed by others

Impact of Microplastics and Nanoplastics in the Aquatic Environment

Microplastic Fate and Impacts in the Environment

Explore related subjects
- Environmental Chemistry
Avoid common mistakes on your manuscript.
Introduction
Global demand for plastics has surged over the past seven decades, resulting in widespread plastic prevalence across modern environmental systems (Campanale et al. 2020 ). This exponential augmentation in plastic production has contributed to a rise in plastic waste deposition across diverse environments (Ebrahimi et al. 2022 ; Spreafico and Russo 2023 ) (Table 1 ) (Fig. S 1 ). According to studies, there are approximately 5.25 trillion bits of plastic floating on the ocean’s surface, although their impact on trophic chains remains unclear (Chaturvedi et al. 2020 ). Plastics release up to 23,600 metric tonnes of dissolved organic carbon annually, of which 60% becomes readily available to microbial populations within 5 days (Romera-Castillo et al. 2018 ). Inadequate handling practices lead plastics to follow diverse pathways in the environment, contributing to significant environmental challenges (Geyer et al. 2017 ; Zhang et al. 2021a , 2021b ). Particular concern surrounds particles with sizes < 5 mm and 0.1 μm due to their longevity, small size, increased surface-to-volume ratio, and their potential to penetrate living cells, eliciting adverse effects (Cole et al. 2013 ; Peixoto et al. 2019 ). Commonly found plastic types in the environment include polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET), and polyethylene (PE) (Güven et al. 2017 ) (Table 1 ).
Under various environmental pressures, such as physical, chemical, or biological influences, plastic materials degrade into smaller particles, forming secondary MPs. Simultaneously, the deliberate inclusion of microscopic plastic particles in industrial processes and household products generates primary MPs in the environment. Based on the size, MPs are categorized as nanoplastics (< 1 μm), microplastics (fragments less than 5 mm in size), mesoplastics (particles measuring between 5 and 25 mm), and macroplastics (pieces exceeding 25 mm in dimension) (Goswami et al. 2020 ; Chauhan et al. 2021 ; Pastorino et al. 2021 ).
MPs are pervasive in various ecological settings, encompassing aquatic, atmospheric, and soil systems (Guo et al. 2020 ; Liu et al. 2022 ). Additionally, MPs have been detected in food products, intensifying concerns (Mei et al. 2020 ). The deterioration of plastics is influenced by their chemical composition and additives, in degradation rates; for instance, a PE bag may take 10–20 years, while a PET bottle can persist for 450–1000 years (Chamas et al. 2020 ). Everyday items like clothing and cosmetics, composed of polyester and polyamide materials, contribute significantly to MPs in the environment (Napper and Thompson 2016 ).
Plastics and MPs released into the environment pose a severe threat to aquatic biota (Li et al. 2021 ). Due to their resemblance to prey, aquatic organisms such as fish, mussels, clams, and others may ingest or absorb MPs, causing physical harm and lethal effects by damaging the digestive system and tissues (Prata et al. 2020 ). Moreover, MPs facilitate the transport of contaminants in the environment, adsorbing substances such as heavy metals, metalloids, per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) and releasing them into various environments. This process poses risks to the digestive and respiratory systems of humans and other animals (Reisser et al. 2014 ; Brennecke et al. 2016 ; Wu et al. 2021 ), especially near metal-releasing sites such as antifouling paint and battery industries (Brennecke et al. 2016 ).
Over the past decade, numerous articles have emerged discussing MPs, encompassing their origins, transport pathways (Wang et al. 2021 ), and their adsorption ability to environmental contaminants (Wang et al. 2018 ; Yu et al. 2021 ) (Fig. S 2 ). Moreover, an increasing number of researchers have sought to explore the interactions between MPs and other environmental contaminants or assessed MPs’ presence in organisms’ tissues. Owing to their diminutive size, MPs are highly susceptible to ingestion by organisms, along with other coupled contaminants. Weber et al. ( 2021 ) tested the ecotoxicological effects of PS fragment MPs in freshwater bivalves and concluded that the tested species were unaffected by PS fragment toxicity. Furthermore, in a recent study, a freshwater bivalve species was exposed to environmental PS (rather than laboratory-based samples). The results confirmed that environmental MPs and NPs triggered stronger immune and detoxification responses in the bivalves (Latchere et al. 2023 ). Nevertheless, it is important to highlight that the mechanisms of action (MoA) and ecotoxicological impacts of contaminants adsorbed to MPs in organisms remain unclear. Thus, elucidating viable pathways for interactions between MPs and various contaminants (organic and inorganic compounds), as well as their MoA in aquatic ecosystems, remains a critical research priority. Additionally, to acquire in-depth knowledge of MPs on the environment, biota, and human beings, it is crucial to analyze and quantify them. Various methods are being employed for sampling and analysis in diverse environmental matrices; nevertheless, there is still no consensus on methodologies tailored to specific environments.
Considering the above facts of MP pollution, the present work focuses on overall aspects of MP pollution starting from its occurrence, behavior, risk, and their characterization analysis. Specific objectives of this work include the following: (1) systematic analysis of factors influencing the genesis of MPs in aquatic systems; (2) investigation of the MoA of various contaminants associated with MPs on aquatic organisms across diverse trophic tiers within the aquatic food web; and (3) discussion of the conceivable risks of MPs on human health; (4) elucidation of effective methodologies for sampling, identification, and quantification of MPs in water and sediment matrices.
The search for relevant publications was conducted through various web resources, utilizing databases like Google Scholar, Web of Science, and Scopus, employing search terms such as “microplastic,” “estimation,” “sediment,” “microalgae,” “contaminant interaction,” and “human health risk.” The search encompassed all available publications until September 2022, revealing only 12 articles on the estimation of microplastics and a single article on the estimation of MPs in sediment (Sundar et al. 2021 ). Only 17 research articles are listed for human health effects, with the initial study on the impacts of MPs on human well-being published in 2018 by Rist et al. (2018). Additionally, VOSviewer (version 1.6.18) was employed for cluster-based analysis, and Web of Science was used for citation analysis (e.g., network map of countries) (Fig. 1 ) and co-occurrence (e.g., density map of keywords) (Fig. 2 ).
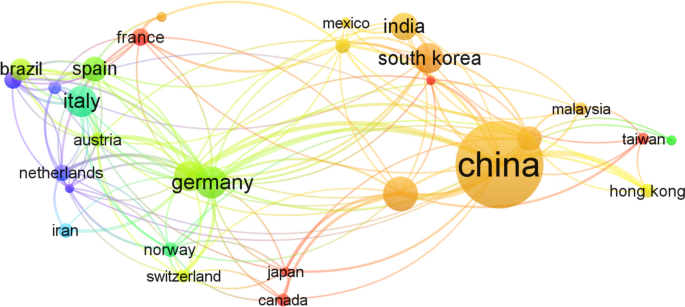
The multinational collaborative network built by co-authorship analyses generated from VOSviewer. The color code represents the clusters and the size of the circle is related to the link strength of all the authors of the specified country. Only the links with a minimum strength of five are displayed
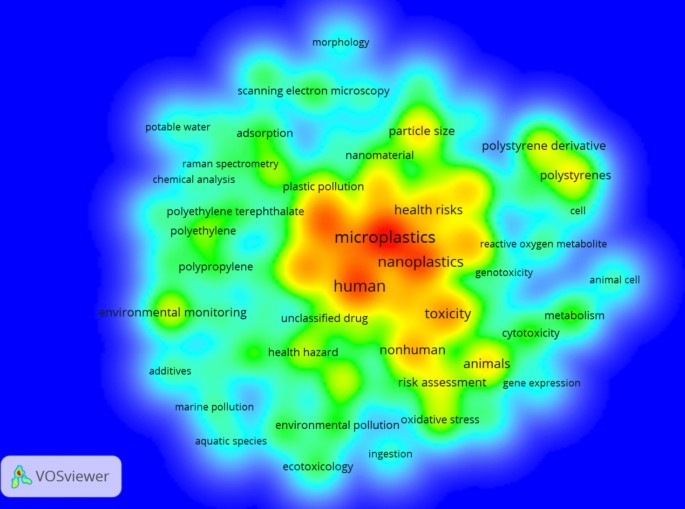
Cluster density analysis of keywords with a word frequency of more than five times. The data come from a literature search with MPs/NPs, ecotoxicity, and human health as the subject terms. The results have been divided into 3 clusters depending on the co-occurrence keywords
Behavior and transformation of plastics into MPs/NPs in aquatic ecosystems
Plastics are highly resistant to natural degradation, and their persistence in the environment leads to gradual transformation into smaller particles, such as MPs and NPs, which have been detected throughout aquatic ecosystems (Plastic Europe, 2019) (Fig. 3 ). These transformations result from various abiotic and biotic mechanisms unique to aquatic environments, including photodegradation, thermal and chemical degradation, mechanical fragmentation, and biological processes. Each mechanism varies in effectiveness depending on polymer type and environmental conditions. This section discusses these processes, focusing on their roles, limitations, and comparative effectiveness in aquatic systems.
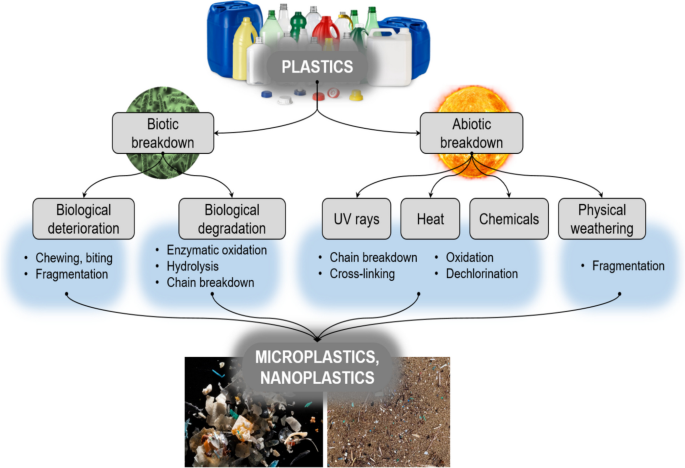
Diagrammatic representation of general processes responsible for the breakdown of plastics in the environment
Solar breakdown of plastics
Solar UV radiation is one of the primary drivers of plastic degradation in aquatic ecosystems, especially at or near the water surface. UV radiation, particularly high-energy UV-B (290–315 nm) and UV-A (315–400 nm) generates free radicals within plastic polymers, initiating photodegradation that leads to the breakdown of polymer chains (Liu et al. 2019b ). This photodegradation process is highly effective for plastics with chromophoric groups that absorb UV light, such as PS and PET. However, many commonly used polymers, including PE and PP, lack natural chromophores, making them more resistant to UV degradation (Fairbrother et al. 2019 ). Impurities or structural imperfections acquired during manufacturing or environmental exposure can act as chromophores for these polymers, increasing their susceptibility to sunlight-driven breakdown (Law 2017 ).
Photodegradation rates are influenced by multiple factors, including polymer type, exposure duration, and geographic location, with higher rates observed in tropical and subtropical regions where UV radiation intensity is greater (Kumar et al. 2020 ). For instance, as carbonyl groups accumulate in PE due to oxidation, they create chromophoric sites that enhance subsequent photo degradability under sustained sunlight exposure, forming smaller organic compounds like aldehydes, ketones, and carboxylic acids (Fairbrother et al. 2019 ).
However, photodegradation primarily occurs on the water surface, as UV light penetration decreases sharply with depth, especially in turbid or polluted waters (Luo et al. 2022 ). This limitation reduces photodegradation effectiveness in deeper aquatic zones, making it less impactful for submerged or suspended plastic particles that are shielded from direct sunlight. In comparison to terrestrial environments, where plastics may be exposed to more intense UV exposure, aquatic photodegradation is limited by light availability, emphasizing the importance of other degradation processes in deep water.
Thermal degradation of plastics in aquatic systems
Thermal degradation, driven by elevated temperatures, plays a key role in the breakdown of plastics, particularly in shallow or coastal aquatic environments exposed to prolonged sunlight. In this process, plastics like PE and PP undergo thermo-oxidative reactions, where heat and oxygen interact to induce radical formation, causing chain scission and, occasionally, cross-linking within the polymer matrix (Zhang et al. 2021a , 2021b ). Similar to photodegradation, these reactions lead to the production of hydroperoxides, which subsequently break down into smaller fragments. The combination of UV and thermal energy can have a synergistic effect, accelerating degradation rates in sunlit coastal areas (Pirsaheb et al. 2020 ).
While elevated temperatures are crucial for thermal degradation, most aquatic environments do not reach the high temperatures required for significant degradation. Typical environmental temperatures in deeper or colder waters fall well below the activation energy needed to trigger thermo-oxidative reactions (Crawford and Quinn 2017 ; Luo et al. 2022 ). As a result, thermal degradation is generally limited to surface or shallow areas and is most effective in warmer climates. In contrast, thermal degradation in terrestrial environments, such as in urban or desert areas with extreme temperatures, may occur more rapidly than in aquatic settings. This environmental limitation reduces the overall impact of thermal degradation in oceans and deep freshwater systems, making it only a supplementary factor in plastic breakdown in aquatic systems.
Chemical breakdown of plastics in aquatic environments
Chemical degradation of plastics in water bodies is influenced by pH, salinity, and the presence of dissolved chemicals and pollutants. Hydrolytic degradation, for instance, is accelerated in aquatic environments with extreme pH levels. Hydrolyzable polymers, like polyamide (PA) and PET, undergo rapid hydrolysis under acidic or basic conditions, leading to the formation of shorter chains and compounds like terephthalic acid and ethylene glycol (Crawford and Quinn 2017 ). For example, PET in low pH environments degrades faster than in neutral pH, where the hydrolysis rate is significantly slower (Hocker et al. 2014 ; Zhang et al. 2021a , 2021b ).
Salinity also contributes to plastic degradation in marine environments. In high-salinity settings, ionic interactions can weaken the structural integrity of plastics, particularly affecting polymer elongation and tensile strength, which predisposes them to mechanical fragmentation (Zhang et al. 2021a , 2021b ). Pollutants like sulfur dioxide (SO₂), nitrogen oxides (NOₓ), and tropospheric ozone (O₃) play a significant role in degradation by generating free radicals that accelerate chain scission in polymer backbones (Weinstein et al. 2016 ). These pollutants also cause secondary reactions that further facilitate plastic breakdown, particularly in coastal regions where pollutant levels are higher due to proximity to human activity (Liu et al. 2019b ).
Despite its relevance, chemical degradation in aquatic environments is typically limited to surface waters where pollutants and UV radiation are most concentrated. In deeper zones, the rate of chemical breakdown slows, resulting in uneven degradation across aquatic environments (Luo et al. 2022 ). Comparatively, terrestrial environments often exhibit more direct interactions between pollutants and plastics, leading to potentially higher degradation rates in heavily polluted urban areas. This contrast highlights the need for further research on how specific pollutants impact plastic degradation in varied aquatic settings.
Mechanical breakdown of plastics in aquatic settings
Mechanical fragmentation is an essential process that reduces larger plastic items into MPs and NPs in aquatic environments. Physical forces, such as wave action, sediment abrasion, and particle collisions, contribute significantly to the breakdown of plastics, especially in rivers, shorelines, and estuarine zones (Thakur 2018 ). Plastics exposed to constant motion in these areas are subject to continuous impact, resulting in fragmentation over time. Mechanical breakdown is especially relevant for polymers with low tensile strength or fracture strain, such as PS and PA, which are more prone to wear from water movement (Salvador Cesa et al. 2017 ).
Mechanical degradation is highly effective in coastal and riverine areas, where tidal movements and wave energy are intense, creating a consistent source of MPs. However, this process is highly dependent on environmental conditions, such as water currents and sediment load, which vary significantly across different aquatic settings (Wagner et al. 2018 ). In calm or deep water, where physical forces are reduced, mechanical fragmentation rates decrease, making the process inconsistent across aquatic environments. By comparison, terrestrial environments may experience direct mechanical degradation through processes like trampling or physical abrasion, which can occur more rapidly than in deep aquatic zones (Luo et al. 2022 ).
Biological degradation of plastics in aquatic systems
Biological degradation in aquatic ecosystems is facilitated by various microorganisms, including bacteria, fungi, and algae, which colonize plastic surfaces and break down plastics through enzymatic action. Hydrolyzable polymers, such as PET and PA, are more susceptible to microbial degradation, as aquatic microbes produce enzymes like lipase, protease, and esterase that catalyze hydrolysis reactions, reducing plastic polymers into bioavailable smaller molecules (Chen et al. 2020a ). Biofilm formation on plastic surfaces in aquatic environments enhances microbial colonization, increasing the degradation rate by providing a stable environment for microbes (Wu et al. 2019b ).
Non-hydrolyzable polymers like PE and PP, however, are more resistant to microbial degradation due to their structural stability and absence of functional groups vulnerable to enzymatic attack (Giyahchi and Moghimi 2023 ). Some microbial communities with lignin-degrading enzymes may facilitate limited degradation of these polymers, but the process is slow and often incomplete in natural settings (Danso et al. 2019 ; Patrício Silva 2022 ). In comparison, terrestrial environments, with varied microbial compositions and environmental conditions, may support different degradation rates and efficiencies depending on soil type, pH, and temperature. The slower biological degradation in aquatic settings underlines the need for additional research on microbial communities specific to water environments to optimize degradation processes for non-hydrolyzable plastics.
Thus, the degradation of plastics in aquatic environments varies widely depending on polymer type, environmental exposure, and the primary degradation mechanisms involved. Table 2 summarizes degradation characteristics for commonly found polymers in aquatic systems, highlighting approximate degradation times, primary degradation mechanisms, and major environmental factors influencing their breakdown.
Each of these processes faces specific limitations within aquatic environments, highlighting the complexity and variability of plastic breakdown across different water systems. In comparison to terrestrial environments, where conditions like direct sunlight, temperature fluctuations, and pollutant exposure may enhance degradation, aquatic systems present unique challenges that slow plastic transformation. Future research should focus on understanding how environmental conditions across diverse aquatic settings influence degradation rates, with a particular emphasis on optimizing conditions for the breakdown of persistent plastic types, such as PE and PP, to reduce the longevity and ecological impact of MPs and NPs in marine and freshwater ecosystems.
Chemical linkage between MPs and organic-inorganic contaminants in the aquatic environment
Organic pollutants.
The primary processes involved in the adsorption of organic pollutants encompass (i) hydrophobic coupling, (ii) partitioning, (iii) electrostatic exchanges, and (iv) non-covalent bonding (Fig. 4 ) (Guo and Wang 2019 ; Liu et al. 2022 ).
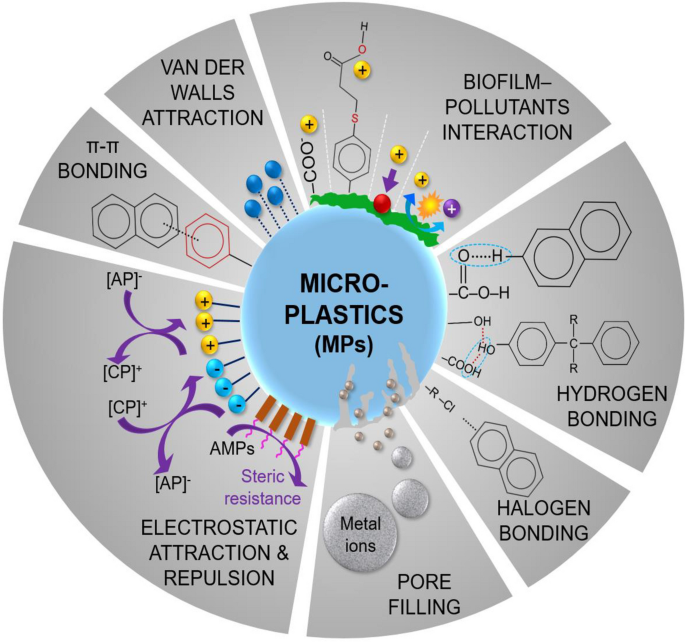
Schematic illustration of possible primary binding mechanisms of organic and inorganic pollutants on MP surface
Hydrophobic coupling between pollutants and MPs
A significant presence of resins in plastics results in pronounced hydrophobicity. Resins are typically categorized into two groups depending on their physical structure: macroporous or macroreticular and highly cross-linked polymers. The primary responsibility for adsorbing organic pollutants lies with MPs carrying these resin types, as they offer substantial surface area and unique functional groups (Chang et al. 2008 ; Ayman.FM et al. 2023 ). Because the majority of organic pollutants are hydrophobic and have poor solubility in water, they may readily adsorb on the surface of MPs (Fu et al. 2020 ). Organic substances with a higher octanol/water partition coefficient ( K ow / K d ) are more prone to adsorb on MPs and vice versa . Bisphenol adsorption on the surface of MPs has revealed a significant linear correlation between uptake capacity and hydrophobicity (Log K ow ) (Wu et al. 2019a ). Furthermore, the adsorption of perflurooctane sulfonamide (FOSA) on the surface of PE MPs has displayed a strong relationship with Log K ow (Liu et al. 2020 ), highlighting the significance of hydrophobicity in capturing the organic pollutants.
Partition effect
The partitioning effect is the dispersion of contaminants between the adjacent aqueous layer surrounding the MPs and the host solution, and it is mostly based on Van der Waals forces following linear adsorption (Liu et al. 2016 ). The adsorption isotherm study for the uptake of aliphatic and aromatic substances on PE MP surface reveals the ~1 n value, specifying the strong linearity and tendency of aromatic substances on MPs through partition coefficient (Hüffer and Hofmann 2016 ). In one investigation, it was confirmed that the n value for Freundlich isotherm in organic drug absorption via PE MPs was equally 1, implying that the isotherm represents linearity (Guo et al. 2019 ). Thus, the partition coefficient is a crucial factor affecting organic chemical adsorption on MPs.
Electrostatic exchange
The electrostatic exchange mechanism is pH-dependent (Guo et al. 2019 ). When the pH of the operational solution exceeds the zero point charge (pH zpc ) of the MPs, the surface of the MPs will be saturated with negative ions, which can attract positively charged ions from organic molecules. If the pH of the working solution surpasses the acid dissociation constant (pKa) of the organic compounds, deprotonation occurs, converting the organic substances to anionic form. Conversion of organic compound surface charges from the cation to anion, causing electrostatic repulsion and preventing sorption on the MP surface (Wu et al. 2019a ). Thus, the electrostatic exchange process involves the electrification of MPs, ion amount, and ionic state of organic substances.
Additional non-covalent bonding
The adsorption process is also influenced by various non-covalent interactions created between organic compounds and MPs, including hydrogen bonding, Van der Walls forces, and π-π bonding, as seen in Fig. 4 (Fu et al. 2021a ). The chemical reaction between hydrophilic organic compounds and ionic PA-MPs is primarily governed by hydrogen bonding (Wu et al. 2019a ). However, organic materials like DDT and PCBs also bond with MP halogen atoms to generate hydrogen bonds (Wu et al. 2019a ). Organic chemicals, such as PAHs and PCBs, are likely to link to PS MPs through π-π bonding (Hüffer and Hofmann 2016 ). Furthermore, π-π bonding facilitates the binding of other organic compounds to the surface of MPs (Fu et al. 2021a ). Van der Walls forces are weak bondings between organic substances and MPs that lack covalent or ionic exchange, mainly occurring between aliphatic polymers.
Inorganic pollutants (metals) adsorption
The MPs can potentially adsorb heavy metals from the nearby environment and can be a vector of metals to aquatic organisms (Bhagat et al. 2021 ; Liu et al. 2022 ). Because of its continual deterioration in the environment, MP surface shape has altered, opening up a variety of routes for heavy metal adsorption (Brennecke et al. 2016 ). Therefore, it is crucial to expose the mechanisms responsible for heavy metal sorption on MPs (Fig. 4 ). The molecular interaction between MP polymer and heavy metal ions majorly determines its adsorption behavior (Zhou et al. 2020 ). Plastic polymer breakdown is hypothesized to trigger metal ions to adhere to the surface of microplastics (MPs) through adsorption. Otherwise, MPs are generally considered unresponsive to metallic ions in water (Fu et al. 2021b ). The sorption behavior of Cd, Co, Cr, Cu, Ni, Pb, and Zn elements on plastic resin pellets sampled from southwest England beaches has been investigated (Holmes et al. 2012 ). According to the findings, ionic sites on the plastic surface provide binding sites for heavy metals, while non-specific interactions are responsible for heavy metal adsorption on neutral zones of MPs. Another study showed that electrostatic interaction between metal cations including Cu 2+ , Ni 2+ , and Zn 2+ and carboxylate ions on the surface of MPs might be responsible for their adsorption (Tang et al. 2022 ). The mechanism of heavy metal adsorption on the surface of MPs could be explained in the following three phases (Guo and Wang 2019 ): (a) heavy metal surface dispersion on the MP surface, (b) the heavy metal pore volume diffusion in MPs, and (c) adsorption on the polar sites.
Research on the adsorption behavior of heavy metals on MPs suggests that the adsorption of Pb 2+ onto nylon MPs transpires spontaneously and involves an endothermic reaction. The carboxylic groups are generated as a result of nylon degradation, providing binding sites for Pb 2+ ions and being regulated by the surface complexation process (Tang et al. 2022 ). Correspondingly, in another research, the mechanism of Pb 2+ adsorption was mostly based on the interaction of carboxyl and hydroxyl groups, as well as an electrostatic exchange reaction (Fu et al. 2021b ). In contrast, one study found that the sorption of Cd 2+ on MPs may be linked to the sequential exchange process (Guo et al. 2020 ). The study indicated that distinct chemical moieties on the exterior of MPs, as well as surface sorption and diffusion effects, are related to Cd 2+ adsorption instead of chemisorption. The impacts of physical weathering of MPs on heavy metal adsorption behavior, such as UV exposure, oxidative reactions, and heat decomposition, have also undergone extensive examination (Rani et al. 2017 ; Kedzierski et al. 2018 ; Liu et al. 2019a ). Usually, oxygen-containing groups formed during weathering have the propensity to enhance the polarity, porosity, hydrophilicity, and ionic charges on the surface of MPs, which affects the adsorption of heavy metals on MPs (Liu et al. 2019a ). The adsorption of heavy metals on PS MPs was examined under different environmental conditions such as UV rays, freshwater, marine water, and air (Mao et al. 2020 ). The results indicated that the adsorption capacity of metal ions on the surface of PS MPs increases with MP aging, which may increase surface roughness and oxygen atom-containing groups that provide a binding site for them. These findings suggested that weathered MPs pose greater harm than pristine MPs of identical polymer composition because environmental influences generate greater surface roughness and oxygen groups, which give an adequate site for metal sorption. Despite these negative impacts of MP transporting heavy metals, there has been relatively limited investigation on the effects of plastics aging under natural environmental settings. The maturation of MPs in the open environment is influenced by a variety of environmental conditions, including the MP residency period and the concentration of heavy metal ions in that specific site, which causes difficulty when comparing existing data. Hence, it is critical to distinguish between the adsorption behavior of metal ions in natural wreathing and artificial aging in the future.
Existing microorganisms form a biofilm around the MPs, altering the surface shape and physicochemical properties of the MPs and increasing heavy metal adsorption (Rummel et al. 2017 ). For 28 days, field research was conducted to investigate the impacts of biofilms in an urbanized estuary using suspended plastic pellets (Richard et al. 2019 ). Upon conclusion of the experiment, it was discovered that elevated levels of metals (Al, Co, Cu, Mn, Pb, and U) on plastic pellets are indicative of a high concentration of metals directly associated with biofilm abundance on MPs. This might be because biofilm formation stimulates the creation of hydrous oxides and active binding sites, as well as the formation of organometallic compounds with metal ions and hydrous oxides, allowing metals to be removed during the separation procedure from biosorption (Rochman et al. 2014 ; Fu et al. 2021b ). However, complicated physical and chemical processes are responsible for the sorption of heavy metals in biofilms. MPs covered with biofilm from a reservoir and a eutrophic urban lake were used for a 1-month experiment to study metal absorption (Guan et al. 2020 ). The research emphasized that biofilm functional moieties, including phenolic, amino, and carboxylic groups, enhance the sorption of heavy metals onto MPs through processes such as cation exchange, electrostatic interchange, and interaction with functional moieties.
On the other hand, UV light has an essential role in creating biofilm on the surface of MPs, which can increase its lifespan and reduce metal adsorption on the MP surface (Richard et al. 2019 ). Although the preceding investigations were conducted over a short period, microbial communities change over time. Each microbial community may exhibit some distinctive behavior toward adsorption mechanisms, which influences metal binding on MP surfaces. To further understand the mechanisms involved in metal ion adsorption on MP surfaces, future studies should focus on long-term processes for microbial development on MPs and their effects on heavy metal bioavailability in the aquatic environment.
Interaction of MPs with aquatic biota
Microalgae play a pivotal role as the main contributors to the aquatic ecosystem and are also affected by MPs which raises a serious question about aquatic ecosystem existence (Liu et al. 2019a ). Because of the size variability of microalgae from micrometers (μm) to millimeters (mm), they have incredible potential to interact with MPs in aqueous conditions.
The mechanism of action (MoA) of MPs in microalgae can shift from the cellular to the communal level, with growth rate suppression being the primary impact (Mao et al. 2018 ; Liu et al. 2019b ; Prata et al. 2020 ; Wang et al. 2020 ). The metabolic impacts include the reduction of photosynthesis and the production of reactive oxygen and nitrogen species (ROS and RNS) that cause oxidative stress in algal cells. The MoA interaction between algal cells and MPs can be explained in two ways: (a) interaction through cell walls or/damaged membrane for direct contact that cause cytotoxicity in algal cells (Liu et al. 2019b ) and (b) MPs affect the photosynthesis process in microalgae through shading effect (limited availability of light) in the water column (Wang et al. 2020 ). MPs with particle sizes of < 1 mm can adhere to the surface of microalgae and influence them in a variety of ways: (i) decrease the cell membrane fluidity; (ii) disturb the cell membrane transportation; (iii) influence the availability of nutrients in algal cells, affecting metabolism and growth; (iv) produces negative effects on cell structure, such as cell membrane damage, and plasmolysis (Mao et al. 2018 ; Liu et al. 2019a ). Furthermore, MP adsorption on algal cells can promote the secretion of protein-rich exopolymeric substances (EPS) that facilitate MPs and biogenic aggregation, along with MP surface alterations, which can influence the fate and colonization of microalgae (Shiu et al. 2020 ). The MPs with particle size < 1 μm can find their way into the algal cell through cell membrane pores (Garrido et al. 2019 ). To summarize, the MoA of MPs on algae is primarily contingent on size, particularly noticeable with larger MPs causing, photosynthetic stress owing to light obstruction. In contrast, small-sized MPs adsorb through the cell membrane, creating biochemical changes affecting cell growth and functions.
Molluscs or aquatic floor organisms
Organisms from group mollusca are good bioindicators of ecotoxicity in aquatic environments. Interestingly, bivalve molluscs have suspension-feeding habits because of their proficiency in sieving substantial water quantities and encapsulating small particles together with MPs, efficiently (Rosa et al. 2018 ). Therefore, MP fibers have been reported in mollusc tissues abundantly, from all over the world.
The uptake of MPs in molluscs can occur either passively or actively. The passive method is a self-occurring process that happens due to their structure and morphology; molluscs exhibit modifications in their feeding organs. The adhesion of sticky particles on the feeding organs is accountable for passive uptake, whereas in the case of active or natural uptake method, selection of particles is triggered by fine alteration in the structure or drifting in feeding organs, such as muscle contractions or movement of cilia or cirri over these organs (Evan Ward and Shumway 2004 ). In the case of bivalves, uptake of MPs followed two pathways: (A) through gills, MPs can be trapped in the gills as they are the foremost organs, and (B) through inhalation via the siphon, MPs can move towards the oral region and subsequently undergo additional translocation and distribution throughout the entire system. Initially, the uptake of MPs is carried out by the microvilli located on the surface of gills. The adhered particles are transported by endocytosis, responsible for the transport of dust and tiny particles in bivalves. The second pathway includes the activity of the ciliary that guides the MPs into the digestive system and facilitates their journey to the primary and secondary ducts of gastrointestinal tubules. The MPs can reach the circulatory system or hemolymph from both routes, through gills as well as by the digestive gland, and circulate in the entire system (Ding et al. 2018 ). However, the retention time of MPs in the gut region is highly dependent on the morphological structure of latero-frontal cilia (Møhlenberg and Riisgård 1978 ). The density of the MPs also plays a crucial role in the retention of MPs; lighter particles can retain for a longer time than heavier ones (Brillant and MacDonald 2000 ). Moreover, rough or irregular particles of MPs can retain for a longer time than smooth ones (Huvet et al. 2016 ). Therefore, the wide distribution of MPs in mollusc tissues indicated a red sign for the aquatic food chain because of their close association with predators and human health.
Owing to their small size and high resemblance with natural food, MPs are highly consumed by fish species, while the presence of MPs has been documented in diverse aquatic organisms such as zooplankton, bivalves, and copepods (Browne et al. 2008 ; Cole et al. 2013 ; Chua et al. 2014 ; Pal and Maiti 2018a , 2018b , 2019a , 2019b , 2020 ) (Table S 1 ), but accumulation and distribution of MPs in commercial and highly consuming organisms are expected to cause exposure risk to human population with possible adverse effects over time.
Ingestion of MPs in fish tissues is dependent on the bioavailability and concentration of pollutants in the surrounding water, physiology of the organism, exposure time, and feeding behavior (Akhbarizadeh et al. 2018 ). The primary route of MPs in fishes is majorly considered through ingestion. The ingested particles are assumed to reside in the intestinal tract, translocated to other parts or fish tissues, and finally eliminated (Fossi et al. 2018 ). Furthermore, MPs have a propensity to attach to the skin of fish and relocate to different organs, including the liver, gills, and muscles (Su et al. 2019 ). A study conducted by Wright and Kelly ( 2017 ) revealed that ultrafine plastic particles can traverse biological membranes and translocate either via the vascular system or lymphatic pathways, demonstrating the distribution of MPs throughout the fish body.
Risk characterization and human health risk assessment
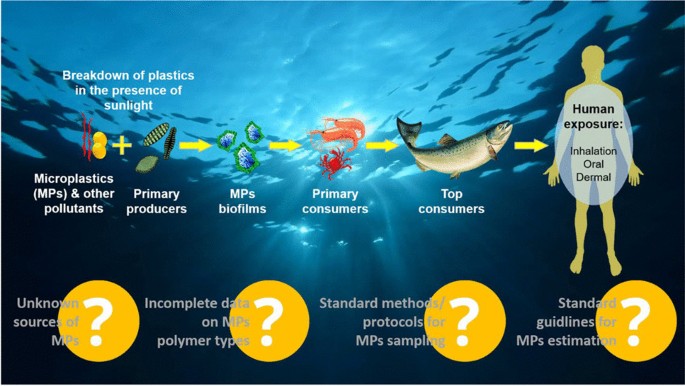
The pervasive presence of MPs in the environment raises substantial health concerns, driven by their persistent nature, bioavailability, and potential effects on human physiology. Human exposure to MPs occurs primarily through ingestion, inhalation, and, potentially through skin contact, as illustrated in Fig. 5 . These exposures impact various bodily systems, including gastrointestinal, respiratory, reproductive, and neurological, prompting investigations into MP health risks and necessary regulatory responses.
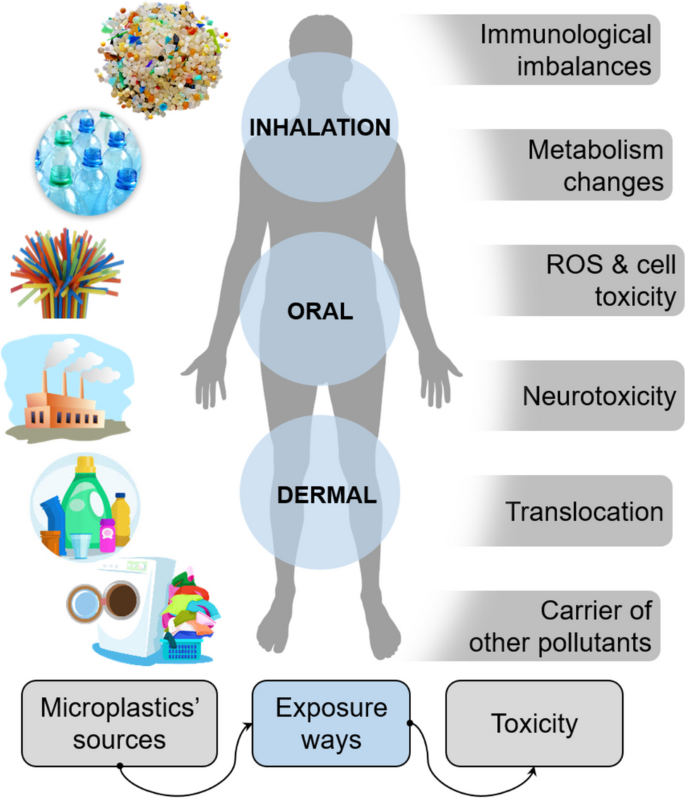
Potential sources, exposure routes, and toxicity routes of MPs/NPs in the human body
Routes of exposure and uptake of MPs/NPs
Ingestion is a primary exposure route, with MPs entering the gastrointestinal (GI) tract via contaminated food and drink. Common sources include seafood, milk, bottled water, and salt (Mason et al. 2018 ; Kutralam-Muniasamy et al. 2020 ; Kwon et al. 2020 ). Animal studies have shown that MPs accumulate in tissues through ingestion and transfer up the food chain, increasing human exposure risk (Cedervall et al. 2012 ). MPs have been detected in 81% of global tap water samples, mainly as fibers < 5 mm (Kosuth et al. 2018 ). In bottled water, particle counts can reach up to 6292 particles per liter (Schymanski et al. 2018 ; Oßmann et al. 2018 ). Infants face higher exposure due to frequent use of plastic containers (Zhang et al. 2021a , 2021b ), with estimated weekly consumption at 0.1–5 g, roughly equivalent to the weight of a credit card (Senathirajah et al. 2021 ).
Airborne MPs, originating from synthetic fibers, tire wear, and industrial sources, can enter the respiratory tract. The extensive alveolar surface (~150 m 2 ) and thin epithelial barrier (~1 μm) allow NPs to penetrate capillaries and enter systemic circulation (Lehner et al. 2019 ). Autopsies have confirmed MP presence in lung tissues, raising concerns about prolonged respiratory exposure (Amato-Lourenço et al. 2021 ). Occupational exposure to airborne MPs, such as polystyrene, is linked to increased styrene metabolites in urine, suggesting biomarker potential for inhalation exposure (Persoons et al. 2018 ).
Skin contact
Skin exposure is less studied but occurs through prolonged contact with MPs in cosmetics and textiles. Certain plastic additives, like bisphenol A (BPA), can induce contact dermatitis, though penetration into deeper skin layers is rare (Aalto-Korte et al. 2003 ). NPs can potentially penetrate the skin via sweat glands, hair follicles, or damaged areas (Yee et al. 2021 ), but they predominantly accumulate in the outermost skin layers. A recent study used a 3D human skin equivalent model and tested the bioavailability of polybrominated diphenyl ether (PBDEs), a well-known chemical additive present in MPs. The study confirmed that more sweaty skin resulted in higher bioavailability of PBDEs from dermal contact than dry skin (Akpojevwe Abafe et al. 2024 ).
Effects of MPs on human organ systems
Gastrointestinal tract.
The GI tract is vulnerable to MPs, which may disrupt gut microbial communities and trigger inflammation. Higher fecal MP concentrations have been observed in patients with inflammatory bowel disease (IBD) compared to healthy controls (Yan et al. 2022 ). Animal studies show that MPs contribute to gut dysbiosis and barrier impairment, potentially leading to metabolic disorders, such as non-alcoholic fatty liver disease (Chen et al. 2022 ). Chronic MP accumulation has also been linked to a heightened risk of colorectal and pancreatic cancers (Oddone 2014 ).
Respiratory tract
The respiratory system is particularly susceptible to MPs and NPs due to its extensive surface area and thin epithelium. Studies in exposed workers have noted links between MP exposure and respiratory conditions like hypersensitivity pneumonitis and obstructive bronchiolitis (Ruder and Bertke 2017 ). Although causality between MP exposure and lung cancer remains inconclusive, autopsy data confirm MPs in lung tissues, indicating risks associated with chronic inhalation (Amato-Lourenço et al. 2021 ).
Skin and allergic reactions
MPs in consumer products, including cosmetics and textiles, may pose dermatological risks. Cases of contact dermatitis have been attributed to plastic additives, including BPA (Rose et al. 2009 ). While direct penetration into deeper skin layers is uncommon, MPs can interact with immune cells in the skin, potentially triggering localized inflammation (Heilig et al. 2011 ).
Reproductive system
Research suggests that MPs can cross biological barriers, including the placenta, potentially affecting fetal development. MPs have been detected on both maternal and fetal sides of human placentas (Ragusa et al. 2021 ). Animal studies indicate that MPs can accumulate in reproductive organs, leading to reduced fertility and gonadal damage, such as ovarian fibrosis and decreased sperm viability (An et al. 2021 ; González-Acedo et al. 2021 ).
Cellular and molecular impacts of MP exposure
This section highlights the combined toxicity of MPs and various environmental pollutants, revealing significant effects on aquatic organisms. Table 3 presents a detailed overview of key studies on synergistic toxicity, including pollutant types, observed biological impacts, mechanisms of toxicity, and potential implications for human health. These interactions often culminate in cellular impacts, where MPs exhibit cytotoxic and genotoxic effects, disrupting cellular functions and posing additional health risks, as discussed below.
Cytotoxicity and genotoxicity
In vitro studies indicate that MPs, particularly polystyrene nanoparticles (PS-NPs), induce cytotoxicity and genotoxicity depending on particle size and surface modifications (Brachner et al. 2020 ). MP exposure leads to DNA damage, sister chromatid exchange, and increased micronuclei formation in cells, especially in occupational settings with high styrene exposure (Teixeira et al. 2010 ). MP bioactivity is further modulated by the protein corona that forms upon contact with biological fluids, varying across physiological states (Ahsan et al. 2018 ).
Oxidative stress and inflammation
MPs are known to induce oxidative stress, where excessive reactive oxygen species (ROS) production surpasses cellular antioxidant defenses, leading to tissue damage (Werder et al. 2018 ). This oxidative stress, combined with inflammation, can exacerbate conditions such as asthma and other inflammatory diseases, especially in settings with prolonged or high-level exposure. Recent researches on cellular exposure to MPs /NPs reveal that these particles can induce various responses, including cytotoxicity, genotoxicity, inflammation, apoptosis, and oxidative stress (Brachner et al. 2020 ). In vitro studies have primarily focused on PS as a model polymer, despite PS not being among the most prevalent plastics globally. This indicates a gap in data for other widely found polymers, especially PET and PVC, which are also significant contributors to human exposure and potential toxicity (Park et al. 2020 ).
Particle size and surface modifications are critical factors influencing cytotoxicity and inflammatory responses. Research has examined particles ranging from nano- to millimeters, often observing stronger biological interactions with smaller particles like NPs. These particles can more easily penetrate cellular membranes, leading to the generation of ROS, which exacerbates oxidative stress and inflammation (Ahsan et al. 2018 ). However, studying these effects presents challenges due to difficulties in isolating and extracting MPs from environmental or biological samples, complicating accurate exposure and dosage assessments. Furthermore, data on long-term exposure effects remain limited, despite the likelihood that plastic particles could bioaccumulate and persist in human tissues over a lifetime, raising concerns about chronic, low-level exposure impacts (Brachner et al. 2020 ).
Upon entering the body, NPs often interact with biomolecules such as proteins, lipids, and sugars, potentially altering their properties. For instance, when NPs contact biological fluids like serum or plasma, they form a “protein corona”—a dynamic layer of adsorbed proteins that modifies their surface characteristics (Park et al. 2020 ). This protein corona varies with physiological factors, such as stress, diet, and health status, potentially influencing how cells recognize these particles, which could alter toxicity. However, the full extent of these effects remains underexplored.
Currently, in vitro studies have not sufficiently explored personalized cellular responses based on specific protein corona compositions, leaving questions about how these variations affect particle behavior and toxicity. More extensive research on diverse polymers and tailored studies on individual physiological conditions is essential to better understand the long-term cellular impacts of MPs and NPs on human health.
Influence of chemical properties of MPs on human health
The interaction between MP chemical composition and human health adds complexity to risk assessment. MPs contain both inherent chemicals (e.g., polymers and additives) and adsorbed environmental pollutants, which can be harmful when ingested or inhaled.
Effects of encapsulated chemicals in MPs
Additives in plastic production, such as halogens, pigments, and flame retardants, can be toxic. These chemicals have been linked to cellular toxicity, genotoxicity, and endocrine disruption (Winiarska et al. 2024 ). For instance, BPA, commonly used in plastic packaging, can mimic natural hormones and disrupt the endocrine system (Olea-Serrano et al. 2002 ) (Table 3 ). The cellular toxicity of MPs, particularly of smaller particles, increases with decreasing size and higher concentrations, making particle characteristics crucial in toxicity assessment (De Souza Machado et al. 2018 ; Winiarska et al. 2024 ).
Effects of chemicals adsorbed on MP surfaces
MPs can adsorb pollutants from their surroundings, concentrating these contaminants on their surfaces by up to 10 6 times the surrounding environment levels (Teuten et al. 2007 ). This adsorption capacity depends on factors like MP size, shape, and surface chemistry, as well as environmental conditions (Fernández et al. 2020 ). MPs facilitate pollutant transport across environments, with PAHs demonstrating strong sorption to MPs in high-salinity waters, thereby enhancing their bioavailability in marine food chains (Sun et al. 2009 ). Experimental studies indicate that MPs and NPs can disrupt red blood cells, with smaller particles causing notable shape changes and reduced cell flexibility (Barbul et al. 2018 ).
In terrestrial ecosystems, MPs and NPs may translocate into plant tissues, entering the food chain. MP ability to transport pollutants, coupled with their persistence in plants like rice, raises concerns about MP mobility in agriculture and potential health impacts from crop contamination (Liu et al. 2022 ) (Fig. 6 ).
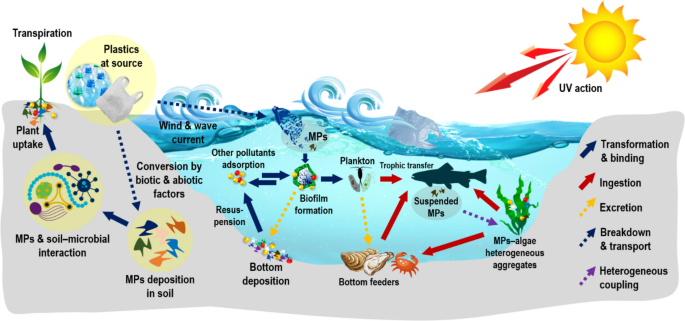
A schematic description of bioaccumulation and effects between MPs/NPs and other organic and inorganic pollutants in the terrestrial and aquatic ecosystem
The exposure pathways, biodistribution, and cellular effects of MPs indicate substantial implications for human health. Ingestion and inhalation represent the primary routes, with MPs detected in multiple tissues, including the gastrointestinal and respiratory tracts, and even placental tissue. Evidence points to potential risks in reproductive, respiratory, and gastrointestinal health, with MPs linked to oxidative stress, inflammation, and possible carcinogenic effects. Reproductive toxicity is particularly concerning, with studies showing placental transfer and gonadal accumulation, necessitating further research into chronic health impacts. Occupational studies underscore respiratory risks, associating styrene and plastic-derived compounds with respiratory disorders and carcinogenesis. Emerging cellular data, including DNA damage and oxidative stress, highlight the urgency for more rigorous studies on long-term, low-dose exposure. Protein corona formation on MPs complicates their cellular interactions, potentially enhancing or modifying their bioactivity and toxicity.
Sampling, identification, and quantification of MPs in water and sediment matrix
Methods for mp sampling from water, neuston, manta nets, and plankton nets.
These nets are widely used, facilitating large-volume water sampling and yielding abundant MPs for further testing. Advantages include ease of use, compatibility for comparative studies across different locations, and capability for high sample yields (Prata et al. 2019 ). However, limitations involve high equipment costs, the need for a boat, potential contamination from vessel or tow ropes, time-intensive processes, and a detection threshold of 333 μm. Another popular method includes the usage of plankton nets, which are advantageous for detecting smaller particles, with a detection limit of 100 μm, and they enable quick, medium-volume sampling (Prata et al. 2019 ; Egessa et al. 2020 ). Despite their ease of use, the equipment is expensive, boat access is required, and static sampling relies on water flow, which may lead to clogging or equipment breakage. Additionally, they sample lower water volumes compared to Manta trawls.
Sieving and pumping
This method requires minimal equipment and does not require a boat, making it accessible for collecting moderate water volumes. However, it is labor-intensive and time-consuming, often involving manual water transfer via buckets. In this approach, approximately 5 L water samples were collected from the Wei River and repeatedly filtered through a fine 75-μm stainless steel sieve to enhance the capture of smaller MPs (Ding et al. 2019 ). In another study, 5 L surface water samples were collected from the Goulburn River, and collected water was filtered through a 20-μm nylon membrane sieve, enhancing the detection of smaller MPs (Nan et al. 2020 ). Compared to the previous field-based 75-μm stainless steel sieve method, this method offers greater precision and contamination control but requires laboratory facilities and specialized equipment.
Pumps facilitate large-volume water sampling with adjustable mesh sizes, offering efficient sample collection despite needing energy, equipment, and facing contamination and portability challenges. Using this approach, about 30 L water samples were pumped through a 330-μm nylon mesh for MP collection from the transboundary Ganga river (Napper et al. 2021 ). In another study, a pumping method with 300 μm and 100 μm sieves was used, and it was found that for monitoring environmentally relevant size fractions of microlitter, a mesh size of 100 μm or smaller is recommended for the Baltic Sea (Setälä et al. 2016 ).
Alternative surface water sampling methods
An alterative water sampling method includes MuMi sampler; 3-D printed hydrodynamic device designed for high-resolution MP sampling, equipped with interchangeable filters; and a flow meter for real-time volume monitoring. Unlike traditional Manta nets, MuMi offers enhanced precision and ease of handling due to its compact design and stabilizing fins, minimizing turbulence. Although it requires a 12 V power source, its real-time data capability makes it ideal for detailed MP analysis (Montoto-Martínez et al. 2022 ). In another study, two newly developed MP sampling systems (New Types I and II) were used for the water sampling, which provides real-time, in situ size fractionation through sequential Y-shaped filters. The New Type I operates in a mobile configuration, sampling as the vessel travels, while New Type II functions in a stationary mode (Du et al. 2022 ). Compared to the MuMi sampler, which allows filtration to 50 μm without sequential fractionation, these systems enable more comprehensive size distribution data across larger spatial areas. However, the new systems require extensive equipment and high power, presenting logistical challenges relative to the MuMi sampler portability and suitability for fine-scale particle analysis.
A study conducted in the Kattegat/Skagerrak area, Denmark, used UFO (Universal Filtering Objects) system for MP collection from surface water. This method allows to collect water samples at 3 m depth, filtering water sequentially through 300-μm and 10-μm stainless steel filters without additional pumping (Gunaalan et al. 2023 ). Unlike MuMi and New Type I/II systems, which target surface waters, the UFO system captures particles from a deeper, turbulent zone, offering high filtration precision and reduced operational complexity. This system is advantageous for studies targeting subsurface MPs with minimal setup, while MuMi and New Type I/II provide broader surface coverage and finer fractionation capabilities.
Sampling of MPs in sediments
Sediment eventually serves as a sink for the pollutants. In the sediment matrices, aquatic biota interacts extensively with MPs; hence, its quantification is of utmost importance. Sampling, extraction, and quantification are the three main aspects of the analytical process that need to be considered for accurately measuring MPs in sediment samples (Hanvey et al. 2017 ). Although there is rapid development in MP research, an inconsistency in the sampling and extraction techniques for MP estimation in sediments can be seen. The lack of universally validated methods has given rise to a variety of analytical approaches, impeding a comprehensive interpretation of current results on a broad scale. The research challenge lies in the complexity of selecting an appropriate method for MP sampling in sediment due to the wide range of available methods. In this section, we aim to consolidate the most pertinent methods for sampling MPs from sediment. Furthermore, the uneven distribution of MPs in sediments necessitates the need to follow a proper sampling method.
Only an appropriate sampling method will help to produce a reliable result since the concentration of MPs differs depending on the sampling area and the depth covered. Therefore, the choice of sampling technique should consider the matrices designated for sampling and the size constraints of the intended MPs (Wang and Wang 2018 ). Usually, the three sampling techniques, viz., selective sampling, bulk sampling, and volume-reduced sampling techniques, are employed for the collection of MP sample from the aquatic environment. Among these, bulk sampling is generally employed for collecting sediment samples (Tsang et al. 2017 ). Samples are mainly collected from the beach, seabed, or superficial depths of the seashore. Collection of samples from beaches is done at a depth of 2–5 cm using stainless spoons, spatula, or hand shovels. Sampling from superficial sediments and seabed requires bottom trawls or specialized equipment, such as a grab sampler and box corer (Prata et al. 2019 ).
Another study developed a Sediment-Microplastic Isolation (SMI) unit, which is a compact, contamination-controlled approach for MP extraction from sediments, integrating sequential filtration and density separation within a single device (Coppock et al. 2017 ). Unlike traditional bulk and selective sampling methods that often require multiple devices and extensive labor, the SMI unit provides efficient particle isolation with minimal cross-contamination risk. Although optimal for laboratory settings with a controlled environment, its limited sediment capacity and dependence on external stirring restrict field applicability. This makes the SMI unit highly suitable for precise lab-based MP analysis, whereas traditional methods remain preferable for larger-scale field sampling.

Separation strategy of MPs from samples
After sampling, MPs need to be separated before quantification and characterization. The most commonly used method for separating MPs is sieving. Sieves of different mesh sizes are used to isolate the MPs from the matrix; however, the method is not sufficient for small-sized MP particles. Different laboratory-based extraction techniques are further required for MP effective separation (Hanvey et al. 2017 ). Density-based separation in combination with filtration is used to isolate small MP particles (< 1 mm) from samples. Different high-density solutions, such as NaCl, NaI, and ZnCl 2 , have been used to make the separation process efficient. Sometimes, the organic matter present in the sample interferes with the accurate estimation of MPs. These organic contents are separated from polymers using a matrix removal process where the samples are digested using different acids, alkalis, and oxidizing agents (Prata et al. 2019 ). Different active and passive separation methods are typically used for smaller-sized MP particles (< 1 μm). Active separation of MPs is achieved using the field flow fractionation (FFF) method, where MP particles are subjected to different magnitudes of externally applied field forces for successful separation. The passive separation utilizes the hydrodynamic chromatography (HDC) method, which uses hydrodynamic and surface forces for particle separation (Fu et al. 2020 ).
MP identification and quantification
Visual inspection method.
The visual inspection technique involves the identification and classification of MPs based on their morphological traits, such as color and dimensions, through direct observation or using a microscope (Table S 2 ). Sorting MPs with the naked eye and optical microscope is the predominant method used for their quantification. The approach is applicable for large-scale sample volumes, particularly in instances where costly analytical instruments are not readily available. However, the method is subjective and may produce large variations between examiners. The probability of errors increases as MPs with lesser diameter are easily miscounted or overlooked. In addition, the morphological changes in the weathered MPs make visual identification even more difficult (Crawford and Quinn 2017 ). To overcome the errors in MP detection in sediments, researchers propose using fluorescence microscopy with Nile Red (NR) staining for MPs (Kukkola et al. 2022 ; Meyers et al. 2022 ; Bakir et al. 2023 ). Alternative techniques including scanning electron microscopy and laser confocal microscopy can assist in mitigating the limitations of visual inspection methods and precisely identify MPs. However, it is still hard to identify MPs of size less than 100 μm using optical microscopes (Song et al. 2015 ) and need more effective and reliable technologies for MP detection.
Thermal analytical method
Pyrolysis gas chromatography-mass spectrometry.
Pyrolysis gas chromatography-mass spectrometry (Pyr-GC–MS) is a detrimental analytical method that identifies the type of MPs based on the analysis of their thermally degraded volatile organic products (Fries et al. 2013 ). Individual MP particles are introduced into a reaction tube where the pyrolysis of the same takes place (Table S 2 ). The vaporous byproducts emitted during the pyrolytic process are subsequently cold-injected and conveyed to the GC column. The characteristic spectra of the pyro-products obtained for unknown samples are compared with the available spectra database made for known polymers that enable the identification of MPs (Käppler et al. 2018 ). The method requires minimal sample pre-treatment and provides detailed information about the chemical composition and other organic additives present in the MPs. Besides, it remains impervious to variations in size, morphology, and additional organic or inorganic impurities linked with the specimen (Dümichen et al. 2017 ). However, the technique is ill-suited for examining extensive sample volumes, as it allows the analysis of only one particle per cycle, which requires around 30 to 100 min. Also, another major limitation of the technique is its inability to provide information related to the particle size of the MPs (Käppler et al. 2018 ).
Thermal extraction desorption gas chromatography-mass spectrometry (TED-GC-MS)
Thermal extraction desorption gas chromatography-mass spectrometry (TED-GC-MS) involves thermo-gravimetric analytical (TGA) solid-phase extraction and thermal desorption gas chromatography-mass spectrometry (TDS-GC–MS). The specimen is initially subjected to heating on a TGA balance at temperatures reaching up to 1000 °C. Following this, the decomposed products undergo adsorption on solid-phase reagents and are then conveyed to a thermal desorption unit (TDU). In TDU, the desorption of deteriorated products is attained through temperature escalation, subsequently separated via a chromatographic column, and subjected to analysis employing mass spectrometry (Dümichen et al. 2017 ). The method saves analysis time for large sample sizes and is suitable for samples that have high mass (up to 100 mg) (Elert et al. 2017 ).
Among the mentioned analytical methods, Py-GC-MS and TED-GC-MS, TED-GC-MS is non-destructive and faster and allows for the analysis of multiple samples in a single run. However, for complex samples such as sediment, Py-GC-MS may be more suitable because it provides more detailed and specific information about the polymer types without overlap between materials. Despite being slower, Py-GC-MS is ideal for identifying the polymer types of MPs in diverse sediment samples.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermal-based analytical method that identifies the polymers based on the chemicals released during the melting of the MP samples. The method is primarily utilized to investigate thermal characteristics of the sample (Rodríguez Chialanza et al. 2018 ). It is one of the simplest and cheapest analytical methods; however, the sample needs pre-treatment before its analysis as the DSC signals vary with the size of the MP particles. The larger particles pose challenges to the analysis due to their higher mass-to-surface area ratio in contrast to the smaller particles, leading to disruptions in the analytical process (Huppertsberg and Knepper 2018 ). In addition, any additives or impurities present in the MPs can influence the transition temperature, thereby resulting in inaccurate analysis results.
Vibration spectral method
Fourier transform infrared spectroscopy.
Fourier transform infrared (FTIR) spectroscopy has emerged as the most commonly used method for the chemical characterization of MPs (Table S 2 ). The unique infrared spectrum generated by the FTIR for a particular chemical bond helps in identifying the unknown material. The different bond compositions of an unknown material generate specific spectra, which are then compared with the known reference spectra for polymer identification (Li et al. 2018 , 2024 ). Besides, the FTIR additionally furnishes comprehensive insights into the physicochemical deterioration of MPs by scrutinizing their oxidative magnitude. Different optimized methods of FTIR, viz., attenuated total reflectance- FTIR (ATR-FTIR), micro-FTIR, and focal plane array- FTIR (FPA-FTIR), have been used for precise and accurate estimation of MPs. ATR-FTIR is generally used in the identification of large-sized (> 500 μm) and irregularly shaped MP particles. However, the processing and separation of MPs from the filters may result in the loss of the smallest MP particles (Shim et al. 2017 ). Unlike ATR-FTIR, micro-FTIR allows the analysis of smaller MP particles (> 10 μm) directly on the filter paper. As a result, the chances of external contamination and the time required for particle sorting are reduced (Chen et al. 2020b ). The incorporation of FPA detectors in FTIR even offers the analysis of the whole surface area of the filter containing MP particles (< 20 μm) with a high spatial resolution (5.5 μm in reflectance and 1 μm in ATR mode). The method is an excellent choice for identifying MPs as it is neither sensitive to the thickness of MPs nor is interfered with by contaminants or filter membranes. Compared with other FTIR techniques, the method is much quicker in detecting MPs; however, the technique is more power- and cost-intensive (Huppertsberg and Knepper 2018 ).
Raman spectroscopy
Raman spectroscopy is another spectral method most widely used in identifying the chemical species of the sample by mapping the frequency shifts of the scattered lights from the sample. Monochromatic laser beams are irradiated onto the MP particles that generate a different frequency of backscattered light as a result of absorption, reflection, or scattering depending on the atomic and molecular structure of the sample (Crawford and Quinn 2017 ). A unique spectrum is produced for each polymer as a consequence of this Raman shift. Micro-Raman enables the identification of MPs as small as 1 μm and, in some cases, even down to 500 nm in high spatial resolution, unlike other conventional spectroscopic techniques (Ribeiro-Claro et al. 2017 ). Besides, it even allows the analysis of moist samples. However, the accuracy of the method is easily influenced due to the existence of additives, colorants, or other contaminants attached to MPs. Also, the low signal-to-noise ratio creates problems in spectrum analysis (Araujo et al. 2018 ).
Among the aforementioned vibration spectral methods, the FTIR method has been predominantly used for MP detection in water samples because it detects vibrational modes of polymer functional groups through infrared absorption, which is minimally affected by water. However, the usage of Raman spectroscopy for MP identification in complex matrices such as sediment is mostly preferred because it relies on the inelastic scattering of photons (Raman effect) to identify polymeric structures. Its ability to resolve smaller MPs (down to 1 μm) and its resistance to interference from pigments or mineral particles in sediment make it highly suitable for such analyses.
Other analytical methods
Scanning electron microscope (sem).
A scanning electron microscope (SEM) uses a high-intensity electron beam to scan the sample (Table S 2 ). The images were produced to provide information about the sample morphology and other surface details at very high magnifications. These high-resolution images are utilized to examine MP surface morphology which helps differentiate them from other organic and inorganic impurities (Wang and Wang 2018 ). In addition, the fractures, grooves, cracks, and pits developed on MPs under weathering can also be easily analyzed using SEM. Besides, the interaction between the sample and electron results in the generation of characteristic X-ray photons, which are specific to certain elements. An energy dispersive spectroscopy (EDS) detector can easily differentiate these X-rays. Hence, in-depth insights into the elemental composition and additional inorganic enhancements within the MPs can be readily acquired through the simultaneous application of SEM and EDS. This process further aids in the differentiation of plastic and non-plastic components (Crawford and Quinn 2017 ). Nevertheless, the method is not suitable for handling a large number of samples due to the considerable time and effort required in sample preparation.
Hyperspectral imaging
Hyperspectral imaging (HSI) is a non-destructive analytical method widely used for detailed sample analysis (Faltynkova and Wagner 2023 ; Goyetche et al. 2023 ; Ali et al. 2024 ). This technique generates spectral information through interactions between incident light of varying wavelengths and the chemical species in a sample (Ye et al. 2022 ), producing spectral signatures that vary depending on the samples’ physical and chemical composition. HSI enables direct visualization of samples, aiding in the identification of polymer types in MPs (MPs) by analyzing their shape, size, and composition (Karlsson et al., 2016). It has been applied to MPs in various environmental samples, including soil (Ai et al. 2022 ), marine sediments (Goyetche et al. 2023 ), seawater (Shan et al. 2019 ), freshwater (Fiore et al. 2022 ), urban wastewater treatment plants (Nguyen et al. 2021 ), and fish tissues (Zhang et al. 2019 ). HSI shows significant potential in MP research, especially when combined with classifiers like support vector machine (SVM) and partial least squares discriminant analysis (PLS-DA), which enhance detection accuracy across a broad MP size range down to 0.1 mm. Further, dimensionality reduction techniques, such as principal component analysis (PCA), improve data handling, enabling efficient processing of high-dimensional spectral data. The successful application of these methods in different wave ranges, from near-infrared (NIR) to short-wave infrared (SWIR), enhances MP detection accuracy (Serranti et al. 2018 ; Shan et al. 2018 ), which is especially useful for mapping polymer types in complex samples and understanding MP distribution in various environments.
Despite its potential, HSI faces challenges in identifying smaller MPs, particularly particles below 0.1 mm, due to weak signal strength and increased noise from surrounding materials. Reflectance imaging, a common technique in HSI, is particularly affected, although preprocessing techniques such as standard normal variate (SNV) and mean centering (MC) have shown some promise in distinguishing MPs within complex mixtures (Shan et al. 2018 ). The non-destructive nature of HSI is advantageous for analyzing MPs in mixed environmental samples, as it preserves sample integrity, enabling a more accurate assessment of MPs in natural settings. Targeted wave ranges, such as 900–1700 nm and 1000–2500 nm, help isolate the spectral signatures of MPs more effectively. However, the complex operation and data processing requirements limit its widespread application, compounded by issues such as low-quality images from electron microscopes and low scanning frame rates (Shan et al. 2019 ). Ultimately, HSI captures spectral signatures based on the unique chemical bonds in MP polymers. In sediment and water samples, MPs reflect light differently due to their composition, such as C-H bonds in polyethylene or polypropylene, allowing differentiation from organic materials. By analyzing these spectral fingerprints, HSI can distinguish MPs from natural particles, even at small sizes, enhancing identification in complex environments. Nonetheless, challenges persist in achieving high resolution and accurate data extraction, as interference from surrounding matrices can limit effectiveness, underscoring the need for further refinement in classifiers and preprocessing techniques to fully harness HSIs capabilities.
Selection of analytical methods based on polymer type
The choice of analytical method is critical for accurate MP identification, particularly when distinguishing between polymer types. FTIR and Raman spectroscopy are preferred for characterizing polymer-specific functional groups, with FTIR well-suited for water samples due to minimal interference and Raman spectroscopy ideal for sediments, as it can resolve smaller MPs (down to 1 μm) and is less affected by pigments or minerals. Thermal methods like Pyr-GC–MS provide detailed polymer composition data without interference from particle shape or size, making them particularly useful for complex matrices, though they require more time per sample. For samples where morphology and surface structure provide essential information, SEM-EDS and HSI offer insights into both elemental composition and surface degradation patterns. Utilizing a combination of these methods allows for robust polymer-type identification across diverse environmental matrices, ensuring more comprehensive MP characterization.
Conclusion and future recommendations
This review systematically explored the pathways of MP formation in aquatic systems, their interactions with environmental contaminants, impacts on biota, and implications for human health risk. Our analysis underscores the pervasive and growing presence of MPs in aquatic environments, driven by factors such as UV radiation, temperature fluctuations, and chemical interactions that facilitate polymer fragmentation and chain scission. These degradation mechanisms are further influenced by environmental variables like low pH and high salinity, accelerating the transformation of larger plastics into MPs.
Advances in sampling and analytical techniques, such as coupling Nile Red staining with fluorescence microscopy, have enhanced the detection of MPs in sediments, although comprehensive studies examining the full range of polymer properties are still required for accurate MP identification and quantification. The adsorption of contaminants on MPs is largely regulated by polymer type and physicochemical conditions (e.g., pH, salinity), with weathering-induced morphological changes increasing the availability of sorption sites. Contaminants bind to MPs through various mechanisms, including hydrophobic and electrostatic interactions, hydrogen bonding, and Van der Waals forces, though laboratory-based findings often lack alignment with the complexities of natural ecosystems.
This review also explores the modes of action of MPs within aquatic food webs, from their interference with photosynthetic processes in microalgae to their bioaccumulation in molluscs and translocation within fish. The buoyancy and density of MPs significantly influence their interaction with pelagic versus benthic organisms, highlighting the importance of tailoring environmental assessments to MP characteristics. The detection of MPs in human stool underscores an unintentional route of human exposure, potentially contributing to a spectrum of health issues, from inflammatory and immune disorders to respiratory and gastrointestinal concerns. Current findings underscore that MPs, particularly in high concentrations or in vulnerable populations, may contribute to adverse health outcomes, yet further studies are needed to clarify these associations.
In alignment with our objectives, this review identifies critical factors influencing MP formation, examines their pathways and modes of action across trophic levels, evaluates their potential risks to human health, and assesses methodological advancements in their identification and quantification in aquatic environments. A more nuanced understanding of MP ecological and health implications will be essential in developing comprehensive risk assessments and management strategies.
Future recommendations
Despite extensive research in this domain, significant gaps persist in the available data. Addressing these knowledge deficiencies requires further exploration of the following issues:
First, advancing cost-effective, user-friendly methods for MP sampling and identification, especially in sediment, water, and biological matrices, is critical for consistent and accessible research. Techniques like fluorescence labelling show promise but remain complex and costly.
In the actual environment, MPs break down in a more complex way than in controlled lab conditions. Thus, for laboratory investigations, integrating diverse degradation techniques is crucial for achieving both consistency and controllability while simulating the environmental breakdown of MPs. Furthermore, it is crucial to understand how MPs interact with other pollutants at different degradation stages, in varying environmental conditions.
Additional research is essential to comprehensively understand the ecotoxicological impacts of MPs under realistic environmental conditions and concentrations. Current toxicity tests employ artificial plastic particles with predetermined size fractions and smooth surfaces on target organisms, which deviates significantly from real-world scenarios. The small size of MPs (or NPs) makes it challenging for researchers to accurately measure their concentration and evaluate their toxicity in organisms, thus turning risk assessment into a tough challenge.
Details regarding the trophic transfer of MPs and linked pollutants in multitrophic aquatic food webs remain constrained. The current focus of trophic transfer studies for MPs primarily involves laboratory-feeding-based secondary food chains, which may not accurately reflect the complexity of real biological systems. In reality, higher trophic level predators possess a stronger capacity to bypass the impact of contaminants compared to lower prey. Consequently, there is a crucial necessity to establish comprehensive experimental conditions for scrutinizing the biological impacts of MPs, especially on apex predators and, consequently, humans.
Most of the studies are focused on the physical characteristics of plastic objects in certain tissues of aquatic biota and the consequent negative environmental impacts. MPs are not likely to induce gastrointestinal blockage in humans; nonetheless, understanding the process of their movement to non-digestive organs may give insight into human health risk assessment.
Data availability
Not applicable
Aalto-Korte K, Alanko K, Henriks-Eckerman M et al (2003) Allergic contact dermatitis from bisphenol A in PVC gloves. Contact Derm 49:202–205. https://doi.org/10.1111/j.0105-1873.2003.0228.x
Article CAS Google Scholar
Ahsan SM, Rao CM, Ahmad MF (2018) Nanoparticle-protein interaction: the significance and role of protein corona. In: Saquib Q, Faisal M, Al-Khedhairy AA, Alatar AA (eds) Cellular and molecular toxicology of nanoparticles. Springer International Publishing, Cham, pp 175–198
Google Scholar
Ai W, Liu S, Liao H et al (2022) Application of hyperspectral imaging technology in the rapid identification of microplastics in farmland soil. Sci Total Environ 807:151030. https://doi.org/10.1016/j.scitotenv.2021.151030
Akhbarizadeh R, Moore F, Keshavarzi B (2018) Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut 232:154–163. https://doi.org/10.1016/j.envpol.2017.09.028
Akpojevwe Abafe O, Harrad S, Abou-Elwafa Abdallah M (2024) Assessment of human dermal absorption of flame retardant additives in polyethylene and polypropylene microplastics using 3D human skin equivalent models. Environ Int 186:108635. https://doi.org/10.1016/j.envint.2024.108635
Ali MA, Lyu X, Ersan MS, Xiao F (2024) Critical evaluation of hyperspectral imaging technology for detection and quantification of microplastics in soil. J Hazard Mater 476:135041. https://doi.org/10.1016/j.jhazmat.2024.135041
Al-Lihaibi S, Al-Mehmadi A, Alarif WM et al (2019) Microplastics in sediments and fish from the Red Sea coast at Jeddah (Saudi Arabia). Environ Chem 16:641. https://doi.org/10.1071/EN19113
Almeda R, Rodriguez-Torres R, Rist S et al (2021) Microplastics do not increase bioaccumulation of petroleum hydrocarbons in Arctic zooplankton but trigger feeding suppression under co-exposure conditions. Sci Total Environ 751:141264. https://doi.org/10.1016/j.scitotenv.2020.141264
Almeida M, Martins MA, Soares AMV et al (2019) Polystyrene nanoplastics alter the cytotoxicity of human pharmaceuticals on marine fish cell lines. Environ Toxicol Pharmacol 69:57–65. https://doi.org/10.1016/j.etap.2019.03.019
Amato-Lourenço LF, Carvalho-Oliveira R, Júnior GR et al (2021) Presence of airborne microplastics in human lung tissue. J Hazard Mater 416:126124. https://doi.org/10.1016/j.jhazmat.2021.126124
An R, Wang X, Yang L et al (2021) Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 449:152665. https://doi.org/10.1016/j.tox.2020.152665
Araujo CF, Nolasco MM, Ribeiro AMP, Ribeiro-Claro PJA (2018) Identification of microplastics using Raman spectroscopy: latest developments and future prospects. Water Res 142:426–440. https://doi.org/10.1016/j.watres.2018.05.060
Ayman.FM F, Taha M, Farghali AA, Abdelhameed RM (2023) Synthesis and applications of porphyrin-based MOFs in removal of pesticide from wastewater: molecular simulations and experimental studies. CrystEngComm 25:6697–6709. https://doi.org/10.1039/D3CE01058A
Article Google Scholar
Bakir A, Doran D, Silburn B et al (2023) A spatial and temporal assessment of microplastics in seafloor sediments: a case study for the UK. Front Mar Sci 9:1093815. https://doi.org/10.3389/fmars.2022.1093815
Barboza LGA, Vieira LR, Branco V et al (2018) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol 195:49–57. https://doi.org/10.1016/j.aquatox.2017.12.008
Barbul A, Singh K, Horev-Azaria L et al (2018) Nanoparticle-decorated erythrocytes reveal that particle size controls the extent of adsorption, cell shape, and cell deformability. ACS Appl Nano Mater 1:3785–3799. https://doi.org/10.1021/acsanm.8b00357
Barrett J, Chase Z, Zhang J et al (2020) Microplastic pollution in deep-sea sediments from the Great Australian Bight. Front Mar Sci 7:576170. https://doi.org/10.3389/fmars.2020.576170
Bergmann M, Wirzberger V, Krumpen T et al (2017) High quantities of microplastic in arctic deep-sea sediments from the HAUSGARTEN observatory. Environ Sci Technol 51:11000–11010. https://doi.org/10.1021/acs.est.7b03331
Bhagat J, Nishimura N, Shimada Y (2021) Toxicological interactions of microplastics/nanoplastics and environmental contaminants: current knowledge and future perspectives. J Hazard Mater 405:123913. https://doi.org/10.1016/j.jhazmat.2020.123913
Bosker T, Guaita L, Behrens P (2018) Microplastic pollution on Caribbean beaches in the Lesser Antilles. Mar Pollut Bull 133:442–447. https://doi.org/10.1016/j.marpolbul.2018.05.060
Brachner A, Fragouli D, Duarte IF et al (2020) Assessment of human health risks posed by nano-and microplastics is currently not feasible. IJERPH 17:8832. https://doi.org/10.3390/ijerph17238832
Brennecke D, Duarte B, Paiva F et al (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195. https://doi.org/10.1016/j.ecss.2015.12.003
Brillant MGS, MacDonald BA (2000) Postingestive selection in the sea scallop, Placopecten magellanicus (Gmelin): the role of particle size and density. J Exp Mar Biol Ecol 253:211–227. https://doi.org/10.1016/S0022-0981(00)00258-6
Bringer A, Thomas H, Dubillot E et al (2021) Subchronic exposure to high-density polyethylene microplastics alone or in combination with chlortoluron significantly affected valve activity and daily growth of the Pacific oyster, Crassostrea gigas. Aquat Toxicol 237:105880. https://doi.org/10.1016/j.aquatox.2021.105880
Browne MA, Dissanayake A, Galloway TS et al (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42:5026–5031. https://doi.org/10.1021/es800249a
Bussolaro D, Wright SL, Schnell S et al (2019) Co-exposure to polystyrene plastic beads and polycyclic aromatic hydrocarbon contaminants in fish gill (RTgill-W1) and intestinal (RTgutGC) epithelial cells derived from rainbow trout (Oncorhynchus mykiss). Environ Pollut 248:706–714. https://doi.org/10.1016/j.envpol.2019.02.066
Campanale C, Massarelli C, Savino I et al (2020) A detailed review study on potential effects of microplastics and additives of concern on human health. IJERPH 17:1212. https://doi.org/10.3390/ijerph17041212
Cedervall T, Hansson L-A, Lard M et al (2012) Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS One 7:e32254. https://doi.org/10.1371/journal.pone.0032254
Chamas A, Moon H, Zheng J et al (2020) Degradation rates of plastics in the environment. ACS Sustainable Chem Eng 8:3494–3511. https://doi.org/10.1021/acssuschemeng.9b06635
Chang C-F, Chang C-Y, Hsu K-E et al (2008) Adsorptive removal of the pesticide methomyl using hypercrosslinked polymers. J Hazard Mater 155:295–304. https://doi.org/10.1016/j.jhazmat.2007.11.057
Chaturvedi S, Yadav BP, Siddiqui NA, Chaturvedi SK (2020) Mathematical modelling and analysis of plastic waste pollution and its impact on the ocean surface. J Ocean Eng Sci 5:136–163. https://doi.org/10.1016/j.joes.2019.09.005
Chauhan JS, Semwal D, Nainwal M et al (2021) Investigation of microplastic pollution in river Alaknanda stretch of Uttarakhand. Environ Dev Sustain 23:16819–16833. https://doi.org/10.1007/s10668-021-01388-y
Chen L, Yuan X, Ye Y et al (2022a) Characteristics and spatiotemporal distribution of microplastics in sediments from a typical mariculture pond area in Qingduizi Bay, North Yellow Sea, China. Mar Pollut Bull 176:113436. https://doi.org/10.1016/j.marpolbul.2022.113436
Chen X, Zhuang J, Chen Q et al (2022) Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol Environ Saf 241:113809. https://doi.org/10.1016/j.ecoenv.2022.113809
Chen Y, Ling Y, Li X et al (2020a) Size-dependent cellular internalization and effects of polystyrene microplastics in microalgae P. helgolandica var. tsingtaoensis and S. quadricauda. J Hazard Mater 399:123092. https://doi.org/10.1016/j.jhazmat.2020.123092
Chen Y, Wen D, Pei J et al (2020b) Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: current status and future prospects. Curr Opin Environ Sci Health 18:14–19. https://doi.org/10.1016/j.coesh.2020.05.004
Chen Z, Ding D, Yu T et al (2022b) Enzymatic degradation behaviors and kinetics of bio-degradable jute/poly (lactic acid) (PLA) composites. Compos Commun 33:101227. https://doi.org/10.1016/j.coco.2022.101227
Chua EM, Shimeta J, Nugegoda D et al (2014) Assimilation of polybrominated diphenyl ethers from microplastics by the marine Amphipod, Allorchestes Compressa . Environ Sci Technol 48:8127–8134. https://doi.org/10.1021/es405717z
Chubarenko I, Esiukova E, Zobkov M, Isachenko I (2022) Microplastics distribution in bottom sediments of the Baltic Sea Proper. Mar Pollut Bull 179:113743. https://doi.org/10.1016/j.marpolbul.2022.113743
Cincinelli A, Scopetani C, Chelazzi D et al (2021) Microplastics in the Black Sea sediments. Sci Total Environ 760:143898. https://doi.org/10.1016/j.scitotenv.2020.143898
Claessens M, Meester SD, Landuyt LV et al (2011) Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull 62:2199–2204. https://doi.org/10.1016/j.marpolbul.2011.06.030
Cole M, Lindeque P, Fileman E et al (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47:6646–6655. https://doi.org/10.1021/es400663f
Coppock RL, Cole M, Lindeque PK et al (2017) A small-scale, portable method for extracting microplastics from marine sediments. Environ Pollut 230:829–837. https://doi.org/10.1016/j.envpol.2017.07.017
Crawford CB, Quinn B (2017) Microplastic pollutants. Elsevier, Amsterdam
Cui Y, Liu M, Selvam S et al (2022) Microplastics in the surface waters of the South China sea and the western Pacific Ocean: different size classes reflecting various sources and transport. Chemosphere 299:134456. https://doi.org/10.1016/j.chemosphere.2022.134456
Danso D, Chow J, Streit WR (2019) Plastics: environmental and biotechnological perspectives on microbial degradation. Appl Environ Microbiol 85:e01095. https://doi.org/10.1128/AEM.01095-19
De Souza Machado AA, Kloas W, Zarfl C et al (2018) Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol 24:1405–1416. https://doi.org/10.1111/gcb.14020
De Villiers S (2018) Quantification of microfibre levels in South Africa’s beach sediments, and evaluation of spatial and temporal variability from 2016 to 2017. Mar Pollut Bull 135:481–489. https://doi.org/10.1016/j.marpolbul.2018.07.058
Devriese LI, De Witte B, Vethaak AD et al (2017) Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): an experimental study. Chemosphere 186:10–16. https://doi.org/10.1016/j.chemosphere.2017.07.121
Ding J-F, Li J-X, Sun C-J et al (2018) Separation and identification of microplastics in digestive system of bivalves. Chin J Anal Chem 46:690–697. https://doi.org/10.1016/S1872-2040(18)61086-2
Ding L, Mao RF, Guo X et al (2019) Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci Total Environ 667:427–434. https://doi.org/10.1016/j.scitotenv.2019.02.332
Du R, Sun X, Lin H, Pan Z (2022) Assessment of manta trawling and two newly-developed surface water microplastic monitoring techniques in the open sea. Sci Total Environ 842:156803. https://doi.org/10.1016/j.scitotenv.2022.156803
Dümichen E, Eisentraut P, Bannick CG et al (2017) Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 174:572–584. https://doi.org/10.1016/j.chemosphere.2017.02.010
Ebrahimi P, Abbasi S, Pashaei R et al (2022) Investigating impact of physicochemical properties of microplastics on human health: a short bibliometric analysis and review. Chemosphere 289:133146. https://doi.org/10.1016/j.chemosphere.2021.133146
Egessa R, Nankabirwa A, Ocaya H, Pabire WG (2020) Microplastic pollution in surface water of Lake Victoria. Sci Total Environ 741:140201. https://doi.org/10.1016/j.scitotenv.2020.140201
Elert AM, Becker R, Duemichen E et al (2017) Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters? Environ Pollut 231:1256–1264. https://doi.org/10.1016/j.envpol.2017.08.074
Eryaşar AR, Gedik K, Şahin A et al (2021) Characteristics and temporal trends of microplastics in the coastal area in the Southern Black Sea over the past decade. Mar Pollut Bull 173:112993. https://doi.org/10.1016/j.marpolbul.2021.112993
Evan Ward J, Shumway SE (2004) Separating the grain from the chaff: particle selection in suspension- and deposit-feeding bivalves. J Exp Mar Biol Ecol 300:83–130. https://doi.org/10.1016/j.jembe.2004.03.002
Fairbrother A, Hsueh H-C, Kim JH et al (2019) Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym Degrad Stab 165:153–160. https://doi.org/10.1016/j.polymdegradstab.2019.05.002
Faltynkova A, Wagner M (2023) Developing and testing a workflow to identify microplastics using near infrared hyperspectral imaging. Chemosphere 336:139186. https://doi.org/10.1016/j.chemosphere.2023.139186
Fernández B, Santos-Echeandía J, Rivera-Hernández JR et al (2020) Mercury interactions with algal and plastic microparticles: comparative role as vectors of metals for the mussel, Mytilus galloprovincialis. J Hazard Mater 396:122739. https://doi.org/10.1016/j.jhazmat.2020.122739
Fiore L, Serranti S, Mazziotti C et al (2022) Classification and distribution of freshwater microplastics along the Italian Po river by hyperspectral imaging. Environ Sci Pollut Res 29:48588–48606. https://doi.org/10.1007/s11356-022-18501-x
Fossi MC, Pedà C, Compa M et al (2018) Bioindicators for monitoring marine litter ingestion and its impacts on Mediterranean biodiversity. Environ Pollut 237:1023–1040. https://doi.org/10.1016/j.envpol.2017.11.019
Fries E, Dekiff JH, Willmeyer J et al (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Processes Impacts 15:1949. https://doi.org/10.1039/c3em00214d
Fu L, Li J, Wang G et al (2021a) Adsorption behavior of organic pollutants on microplastics. Ecotoxicol Environ Saf 217:112207. https://doi.org/10.1016/j.ecoenv.2021.112207
Fu Q, Tan X, Ye S et al (2021b) Mechanism analysis of heavy metal lead captured by natural-aged microplastics. Chemosphere 270:128624. https://doi.org/10.1016/j.chemosphere.2020.128624
Fu W, Min J, Jiang W et al (2020) Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci Total Environ 721:137561. https://doi.org/10.1016/j.scitotenv.2020.137561
Garrido S, Linares M, Campillo JA, Albentosa M (2019) Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicol Environ Saf 173:103–109. https://doi.org/10.1016/j.ecoenv.2019.02.020
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782. https://doi.org/10.1126/sciadv.1700782
Giyahchi M, Moghimi H (2023) The role and application of microbial enzymes in microplastics’ bioremediation: available and future perspectives. In: Thakur S, Singh L (eds) ACS symposium series. American Chemical Society, Washington, DC, pp 33–56
González-Acedo A, García-Recio E, Illescas-Montes R et al (2021) Evidence from in vitro and in vivo studies on the potential health repercussions of micro- and nanoplastics. Chemosphere 280:130826. https://doi.org/10.1016/j.chemosphere.2021.130826
Goswami P, Vinithkumar NV, Dharani G (2020) First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar Pollut Bull 155:111163. https://doi.org/10.1016/j.marpolbul.2020.111163
Goswami P, Vinithkumar NV, Dharani G (2021) Microplastics particles in seafloor sediments along the Arabian Sea and the Andaman Sea continental shelves: first insight on the occurrence, identification, and characterization. Mar Pollut Bull 167:112311. https://doi.org/10.1016/j.marpolbul.2021.112311
Goyetche R, Kortazar L, Amigo JM (2023) Issues with the detection and classification of microplastics in marine sediments with chemical imaging and machine learning. TrAC Trends Anal Chem 166:117221. https://doi.org/10.1016/j.trac.2023.117221
Guan J, Qi K, Wang J et al (2020) Microplastics as an emerging anthropogenic vector of trace metals in freshwater: significance of biofilms and comparison with natural substrates. Water Res 184:116205. https://doi.org/10.1016/j.watres.2020.116205
Gunaalan K, Almeda R, Lorenz C et al (2023) Abundance and distribution of microplastics in surface waters of the Kattegat/ Skagerrak (Denmark). Environ Pollut 318:120853. https://doi.org/10.1016/j.envpol.2022.120853
Guo J-J, Huang X-P, Xiang L et al (2020) Source, migration and toxicology of microplastics in soil. Environ Int 137:105263. https://doi.org/10.1016/j.envint.2019.105263
Guo X, Liu Y, Wang J (2019) Sorption of sulfamethazine onto different types of microplastics: a combined experimental and molecular dynamics simulation study. Mar Pollut Bull 145:547–554. https://doi.org/10.1016/j.marpolbul.2019.06.063
Guo X, Wang J (2019) The chemical behaviors of microplastics in marine environment: a review. Mar Pollut Bull 142:1–14. https://doi.org/10.1016/j.marpolbul.2019.03.019
Güven O, Gökdağ K, Jovanović B, Kıdeyş AE (2017) Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ Pollut 223:286–294. https://doi.org/10.1016/j.envpol.2017.01.025
Hanvey JS, Lewis PJ, Lavers JL et al (2017) A review of analytical techniques for quantifying microplastics in sediments. Anal Methods 9:1369–1383. https://doi.org/10.1039/C6AY02707E
Heilig S, Adams DR, Zaenglein AL (2011) Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 28:587–590. https://doi.org/10.1111/j.1525-1470.2010.01211.x
Hocker S, Rhudy AK, Ginsburg G, Kranbuehl DE (2014) Polyamide hydrolysis accelerated by small weak organic acids. Polymer 55:5057–5064. https://doi.org/10.1016/j.polymer.2014.08.010
Holmes LA, Turner A, Thompson RC (2012) Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut 160:42–48. https://doi.org/10.1016/j.envpol.2011.08.052
Hüffer T, Hofmann T (2016) Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ Pollut 214:194–201. https://doi.org/10.1016/j.envpol.2016.04.018
Huppertsberg S, Knepper TP (2018) Instrumental analysis of microplastics—benefits and challenges. Anal Bioanal Chem 410:6343–6352. https://doi.org/10.1007/s00216-018-1210-8
Huvet A, Paul-Pont I, Fabioux C et al (2016) Reply to Lenz et al.: quantifying the smallest microplastics is the challenge for a comprehensive view of their environmental impacts. Proc Natl Acad Sci USA 113. https://doi.org/10.1073/pnas.1607221113
Käppler A, Fischer M, Scholz-Böttcher BM et al (2018) Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal Bioanal Chem 410:5313–5327. https://doi.org/10.1007/s00216-018-1185-5
Kedzierski M, D’Almeida M, Magueresse A et al (2018) Threat of plastic ageing in marine environment. Adsorption/desorption of micropollutants. Mar Pollut Bull 127:684–694. https://doi.org/10.1016/j.marpolbul.2017.12.059
Kosuth M, Mason SA, Wattenberg EV (2018) Anthropogenic contamination of tap water, beer, and sea salt. PLoS One 13:e0194970. https://doi.org/10.1371/journal.pone.0194970
Kukkola AT, Senior G, Maes T et al (2022) A large-scale study of microplastic abundance in sediment cores from the UK continental shelf and slope. Mar Pollut Bull 178:113554. https://doi.org/10.1016/j.marpolbul.2022.113554
Kumar M, Xiong X, He M et al (2020) Microplastics as pollutants in agricultural soils. Environ Pollut 265:114980. https://doi.org/10.1016/j.envpol.2020.114980
Kutralam-Muniasamy G, Pérez-Guevara F, Elizalde-Martínez I, Shruti VC (2020) Branded milks – are they immune from microplastics contamination? Sci Total Environ 714:136823. https://doi.org/10.1016/j.scitotenv.2020.136823
Kwon J-H, Kim J-W, Pham TD et al (2020) Microplastics in food: a review on analytical methods and challenges. IJERPH 17:6710. https://doi.org/10.3390/ijerph17186710
Latchere O, Roman C, Métais I et al (2023) Toxicity assessment of environmental MPs and NPs and polystyrene NPs on the bivalve Corbicula fluminea using a multi-marker approach. Comp Biochem Physiol Part C Toxicol Pharmacol 273:109714. https://doi.org/10.1016/j.cbpc.2023.109714
Law KL (2017) Plastics in the marine environment. Annu Rev Mar Sci 9:205–229. https://doi.org/10.1146/annurev-marine-010816-060409
Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B (2019) Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol 53:1748–1765. https://doi.org/10.1021/acs.est.8b05512
Li J, Chapman EC, Shi H, Rotchell JM (2020) PVC does not influence cadmium uptake or effects in the mussel (Mytilus edulis). Bull Environ Contam Toxicol 104:315–320. https://doi.org/10.1007/s00128-020-02789-x
Li J, Liu H, Paul Chen J (2018) Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/j.watres.2017.12.056
Li Y, Zhang C, Tian Z et al (2024) Identification and quantification of nanoplastics (20–1000 nm) in a drinking water treatment plant using AFM-IR and Pyr-GC/MS. J Hazard Mater 463:132933. https://doi.org/10.1016/j.jhazmat.2023.132933
Li Z, Li Q, Li R et al (2021) The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ Sci Pollut Res 28:16042–16053. https://doi.org/10.1007/s11356-020-11702-2
Liu K, Wang X, Wei N et al (2019a) Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: implications for human health. Environ Int 132:105127. https://doi.org/10.1016/j.envint.2019.105127
Liu L, Fokkink R, Koelmans AA (2016) Sorption of polycyclic aromatic hydrocarbons to polystyrene nanoplastic. Environ Toxicol Chem 35:1650–1655. https://doi.org/10.1002/etc.3311
Liu P, Qian L, Wang H et al (2019b) New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ Sci Technol 53:3579–3588. https://doi.org/10.1021/acs.est.9b00493
Liu S, Huang J, Zhang W et al (2022) Microplastics as a vehicle of heavy metals in aquatic environments: a review of adsorption factors, mechanisms, and biological effects. J Environ Manag 302:113995. https://doi.org/10.1016/j.jenvman.2021.113995
Liu Z, Qin Q, Hu Z et al (2020) Adsorption of chlorophenols on polyethylene terephthalate microplastics from aqueous environments: kinetics, mechanisms and influencing factors. Environ Pollut 265:114926. https://doi.org/10.1016/j.envpol.2020.114926
Lorenz C, Roscher L, Meyer MS et al (2019) Spatial distribution of microplastics in sediments and surface waters of the southern North Sea. Environ Pollut 252:1719–1729. https://doi.org/10.1016/j.envpol.2019.06.093
Luo H, Liu C, He D et al (2022) Environmental behaviors of microplastics in aquatic systems: a systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J Hazard Mater 423:126915. https://doi.org/10.1016/j.jhazmat.2021.126915
Manbohi A, Mehdinia A, Rahnama R, Dehbandi R (2021b) Microplastic pollution in inshore and offshore surface waters of the southern Caspian Sea. Chemosphere 281:130896. https://doi.org/10.1016/j.chemosphere.2021.130896
Manbohi A, Mehdinia A, Rahnama R et al (2021a) Spatial distribution of microplastics in sandy beach and inshore-offshore sediments of the southern Caspian Sea. Mar Pollut Bull 169:112578. https://doi.org/10.1016/j.marpolbul.2021.112578
Mao R, Lang M, Yu X et al (2020) Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J Hazard Mater 393:122515. https://doi.org/10.1016/j.jhazmat.2020.122515
Mao Y, Ai H, Chen Y et al (2018) Phytoplankton response to polystyrene microplastics: perspective from an entire growth period. Chemosphere 208:59–68. https://doi.org/10.1016/j.chemosphere.2018.05.170
Mason SA, Welch VG, Neratko J (2018) Synthetic polymer contamination in bottled water. Front Chem 6:407. https://doi.org/10.3389/fchem.2018.00407
Matsuguma Y, Takada H, Kumata H et al (2017) Microplastics in sediment cores from Asia and Africa as indicators of temporal trends in plastic pollution. Arch Environ Contam Toxicol 73:230–239. https://doi.org/10.1007/s00244-017-0414-9
Mei W, Chen G, Bao J et al (2020) Interactions between microplastics and organic compounds in aquatic environments: a mini review. Sci Total Environ 736:139472. https://doi.org/10.1016/j.scitotenv.2020.139472
Meyers N, Catarino AI, Declercq AM et al (2022) Microplastic detection and identification by Nile red staining: towards a semi-automated, cost- and time-effective technique. Sci Total Environ 823:153441. https://doi.org/10.1016/j.scitotenv.2022.153441
Mishra A, Buhhalko N, Lind K et al (2022) Spatiotemporal variability of microplastics in the Eastern Baltic Sea. Front Mar Sci 9:875984. https://doi.org/10.3389/fmars.2022.875984
Mistri M, Scoponi M, Granata T et al (2020) Types, occurrence and distribution of microplastics in sediments from the northern Tyrrhenian Sea. Mar Pollut Bull 153:111016. https://doi.org/10.1016/j.marpolbul.2020.111016
Møhlenberg F, Riisgård HU (1978) Efficiency of particle retention in 13 species of suspension feeding bivalves. Ophelia 17:239–246. https://doi.org/10.1080/00785326.1978.10425487
Montoto-Martínez T, Meléndez-Díez C, Melián-Ramírez A et al (2022) Comparison between the traditional Manta net and an innovative device for microplastic sampling in surface marine waters. Mar Pollut Bull 185:114237. https://doi.org/10.1016/j.marpolbul.2022.114237
Mu J, Qu L, Jin F et al (2019) Abundance and distribution of microplastics in the surface sediments from the northern Bering and Chukchi Seas. Environ Pollut 245:122–130. https://doi.org/10.1016/j.envpol.2018.10.097
Nan B, Su L, Kellar C et al (2020) Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ Pollut 259:113865. https://doi.org/10.1016/j.envpol.2019.113865
Napper IE, Baroth A, Barrett AC et al (2021) The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ Pollut 274:116348. https://doi.org/10.1016/j.envpol.2020.116348
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull 112:39–45. https://doi.org/10.1016/j.marpolbul.2016.09.025
Nguyen NB, Kim M-K, Le QT et al (2021) Spectroscopic analysis of microplastic contaminants in an urban wastewater treatment plant from Seoul, South Korea. Chemosphere 263:127812. https://doi.org/10.1016/j.chemosphere.2020.127812
Oddone E (2014) Occupational exposures and colorectal cancers: a quantitative overview of epidemiological evidence. WJG 20:12431. https://doi.org/10.3748/wjg.v20.i35.12431
Olea-Serrano N, Fernández-Cabrera MF, Pulgar-Encinas R, Olea-Serrano F (2002) Endocrine disrupting chemicals: harmful substances and how to test them. Cad Saúde Pública 18:489–494. https://doi.org/10.1590/S0102-311X2002000200013
Oßmann BE, Sarau G, Holtmannspötter H et al (2018) Small-sized microplastics and pigmented particles in bottled mineral water. Water Res 141:307–316. https://doi.org/10.1016/j.watres.2018.05.027
Pal D, Maiti SK (2018a) Seasonal variation of heavy metals in water, sediment, and highly consumed cultured fish (Labeo rohita and Labeo bata) and potential health risk assessment in aquaculture pond of the coal city, Dhanbad (India). Environ Sci Pollut Res 25:12464–12480. https://doi.org/10.1007/s11356-018-1424-5
Pal D, Maiti SK (2018b) Heavy metal speciation, leaching and toxicity status of a tropical rain-fed river Damodar, India. Environ Geochem Health 40:2303–2324. https://doi.org/10.1007/s10653-018-0097-9
Pal D, Maiti SK (2019a) Evaluation of potential human health risks from toxic metals via consumption of cultured fish Species Labeo rohita: a case study from an urban aquaculture pond. Expo Health 11:33–46. https://doi.org/10.1007/s12403-017-0264-8
Pal D, Maiti SK (2019b) Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J Clean Prod 212:986–996. https://doi.org/10.1016/j.jclepro.2018.12.087
Pal D, Maiti SK (2020) An approach to counter sediment toxicity by immobilization of heavy metals using waste fish scale derived biosorbent. Ecotoxicol Environ Saf 187:109833. https://doi.org/10.1016/j.ecoenv.2019.109833
Park JW, Lee SJ, Hwang DY, Seo S (2020) Recent purification technologies and human health risk assessment of microplastics. Materials 13:5196. https://doi.org/10.3390/ma13225196
Pastorino P, Nocita A, Ciccotelli V et al (2021) Health risk assessment of potentially toxic elements, persistence of NDL-PCB, PAHs, and microplastics in the translocated edible freshwater Sinotaia quadrata (Gasteropoda, Viviparidae): a case study from the Arno River Basin (Central Italy). Expo Health 13:583–596. https://doi.org/10.1007/s12403-021-00404-w
Patrício Silva AL (2022) Role of microorganisms in eco-remediation. In: Rocha-Santos T, Costa MF, Mouneyrac C (eds) Handbook of Microplastics in the Environment. Springer International Publishing, Cham, pp 1237–1275
Chapter Google Scholar
Peixoto D, Pinheiro C, Amorim J et al (2019) Microplastic pollution in commercial salt for human consumption: a review. Estuar Coast Shelf Sci 219:161–168. https://doi.org/10.1016/j.ecss.2019.02.018
Persoons R, Richard J, Herve C et al (2018) Biomonitoring of styrene occupational exposures: biomarkers and determinants. Toxicol Lett 298:99–105. https://doi.org/10.1016/j.toxlet.2018.06.1211
Pirsaheb M, Hossini H, Makhdoumi P (2020) Review of microplastic occurrence and toxicological effects in marine environment: experimental evidence of inflammation. Process Saf Environ Prot 142:1–14. https://doi.org/10.1016/j.psep.2020.05.050
Pojar I, Stănică A, Stock F et al (2021) Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea. Sci Rep 11:2000. https://doi.org/10.1038/s41598-021-81724-4
Prata JC, Da Costa JP, Duarte AC, Rocha-Santos T (2019) Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends Anal Chem 110:150–159. https://doi.org/10.1016/j.trac.2018.10.029
Prata JC, Da Costa JP, Lopes I et al (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Ragusa A, Svelato A, Santacroce C et al (2021) Plasticenta: first evidence of microplastics in human placenta. Environ Int 146:106274. https://doi.org/10.1016/j.envint.2020.106274
Rangel-Buitrago N, Arroyo-Olarte H, Trilleras J et al (2021) Microplastics pollution on Colombian Central Caribbean beaches. Mar Pollut Bull 170:112685. https://doi.org/10.1016/j.marpolbul.2021.112685
Rani M, Shim WJ, Jang M et al (2017) Releasing of hexabromocyclododecanes from expanded polystyrenes in seawater -field and laboratory experiments. Chemosphere 185:798–805. https://doi.org/10.1016/j.chemosphere.2017.07.042
Ranjani M, Veerasingam S, Venkatachalapathy R et al (2021) Assessment of potential ecological risk of microplastics in the coastal sediments of India: a meta-analysis. Mar Pollut Bull 163:111969. https://doi.org/10.1016/j.marpolbul.2021.111969
Rehse S, Kloas W, Zarfl C (2018) Microplastics reduce short-term effects of environmental contaminants. Part I: Effects of bisphenol A on freshwater zooplankton are lower in presence of polyamide particles. IJERPH 15:280. https://doi.org/10.3390/ijerph15020280
Reisser J, Shaw J, Hallegraeff G et al (2014) Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates. PLoS One 9:e100289. https://doi.org/10.1371/journal.pone.0100289
Ribeiro-Claro P, Nolasco MM, Araújo C (2017) Characterization of microplastics by Raman spectroscopy. In: Comprehensive Analytical Chemistry. Elsevier, pp 119–151
Richard H, Carpenter EJ, Komada T et al (2019) Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci Total Environ 683:600–608. https://doi.org/10.1016/j.scitotenv.2019.04.331
Rochman CM, Hentschel BT, Teh SJ (2014) Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS One 9:e85433. https://doi.org/10.1371/journal.pone.0085433
Rodríguez Chialanza M, Sierra I, Pérez Parada A, Fornaro L (2018) Identification and quantitation of semi-crystalline microplastics using image analysis and differential scanning calorimetry. Environ Sci Pollut Res 25:16767–16775. https://doi.org/10.1007/s11356-018-1846-0
Romera-Castillo C, Pinto M, Langer TM et al (2018) Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat Commun 9:1430. https://doi.org/10.1038/s41467-018-03798-5
Rosa M, Ward JE, Shumway SE (2018) Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. J Shellfish Res 37:727–746. https://doi.org/10.2983/035.037.0405
Rose RF, Lyons P, Horne H, Mark Wilkinson S (2009) A review of the materials and allergens in protective gloves. Contact Derm 61:129–137. https://doi.org/10.1111/j.1600-0536.2009.01580.x
Ruder AM, Bertke SJ (2017) Cancer incidence among boat-building workers exposed to styrene. American J Industrial Med 60:651–657. https://doi.org/10.1002/ajim.22735
Ruiz-Compean P, Ellis J, Cúrdia J et al (2017) Baseline evaluation of sediment contamination in the shallow coastal areas of Saudi Arabian Red Sea. Mar Pollut Bull 123:205–218. https://doi.org/10.1016/j.marpolbul.2017.08.059
Rummel CD, Jahnke A, Gorokhova E et al (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4:258–267. https://doi.org/10.1021/acs.estlett.7b00164
Salvador Cesa F, Turra A, Baruque-Ramos J (2017) Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci Total Environ 598:1116–1129. https://doi.org/10.1016/j.scitotenv.2017.04.172
Santos D, Luzio A, Matos C et al (2021) Microplastics alone or co-exposed with copper induce neurotoxicity and behavioral alterations on zebrafish larvae after a subchronic exposure. Aquat Toxicol 235:105814. https://doi.org/10.1016/j.aquatox.2021.105814
Schymanski D, Goldbeck C, Humpf H-U, Fürst P (2018) Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res 129:154–162. https://doi.org/10.1016/j.watres.2017.11.011
Senathirajah K, Attwood S, Bhagwat G et al (2021) Estimation of the mass of microplastics ingested – a pivotal first step towards human health risk assessment. J Hazard Mater 404:124004. https://doi.org/10.1016/j.jhazmat.2020.124004
Serranti S, Palmieri R, Bonifazi G, Cózar A (2018) Characterization of microplastic litter from oceans by an innovative approach based on hyperspectral imaging. Waste Manag 76:117–125. https://doi.org/10.1016/j.wasman.2018.03.003
Setälä O, Magnusson K, Lehtiniemi M, Norén F (2016) Distribution and abundance of surface water microlitter in the Baltic Sea: a comparison of two sampling methods. Mar Pollut Bull 110:177–183. https://doi.org/10.1016/j.marpolbul.2016.06.065
Shan J, Zhao J, Liu L et al (2018) A novel way to rapidly monitor microplastics in soil by hyperspectral imaging technology and chemometrics. Environ Pollut 238:121–129. https://doi.org/10.1016/j.envpol.2018.03.026
Shan J, Zhao J, Zhang Y et al (2019) Simple and rapid detection of microplastics in seawater using hyperspectral imaging technology. Anal Chim Acta 1050:161–168. https://doi.org/10.1016/j.aca.2018.11.008
Shim WJ, Hong SH, Eo SE (2017) Identification methods in microplastic analysis: a review. Anal Methods 9:1384–1391. https://doi.org/10.1039/C6AY02558G
Shiu R-F, Vazquez CI, Chiang C-Y et al (2020) Nano- and microplastics trigger secretion of protein-rich extracellular polymeric substances from phytoplankton. Sci Total Environ 748:141469. https://doi.org/10.1016/j.scitotenv.2020.141469
Song YK, Hong SH, Jang M et al (2015) A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar Pollut Bull 93:202–209. https://doi.org/10.1016/j.marpolbul.2015.01.015
Spreafico C, Russo D (2023) Investigating the evolution of the technologies for collecting microplastics. J Environ Manag 326:116710. https://doi.org/10.1016/j.jenvman.2022.116710
Su L, Deng H, Li B et al (2019) The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J Hazard Mater 365:716–724. https://doi.org/10.1016/j.jhazmat.2018.11.024
Sun H, Wu W, Wang L (2009) Phenanthrene partitioning in sediment–surfactant–fresh/saline water systems. Environ Pollut 157:2520–2528. https://doi.org/10.1016/j.envpol.2009.03.012
Sundar S, Chokkalingam L, Roy PD, Usha T (2021) Estimation of microplastics in sediments at the southernmost coast of India (Kanyakumari). Environ Sci Pollut Res 28:18495–18500. https://doi.org/10.1007/s11356-020-10333-x
Tang X, Zhang B, Fan C et al (2022) The presence of cationic polyacrylamide attenuated the toxicity of polyvinyl chloride microplastics to anaerobic digestion of waste activated sludge. Chem Eng J 427:131442. https://doi.org/10.1016/j.cej.2021.131442
Teixeira JP, Gaspar J, Coelho P et al (2010) Cytogenetic and DNA damage on workers exposed to styrene. Mutagenesis 25:617–621. https://doi.org/10.1093/mutage/geq049
Teuten EL, Rowland SJ, Galloway TS, Thompson RC (2007) Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol 41:7759–7764. https://doi.org/10.1021/es071737s
Thakur VK (ed) (2018) Biopolymer grafting: synthesis and properties. Elsevier, Oxford, England Cambridge, Massachusetts
Tran Nguyen QA, Nguyen HNY, Strady E et al (2020) Characteristics of microplastics in shoreline sediments from a tropical and urbanized beach (Da Nang, Vietnam). Mar Pollut Bull 161:111768. https://doi.org/10.1016/j.marpolbul.2020.111768
Tsang YY, Mak CW, Liebich C et al (2017) Microplastic pollution in the marine waters and sediments of Hong Kong. Mar Pollut Bull 115:20–28. https://doi.org/10.1016/j.marpolbul.2016.11.003
Urban-Malinga B, Zalewski M, Jakubowska A et al (2020) Microplastics on sandy beaches of the southern Baltic Sea. Mar Pollut Bull 155:111170. https://doi.org/10.1016/j.marpolbul.2020.111170
Wagner S, Hüffer T, Klöckner P et al (2018) Tire wear particles in the aquatic environment - A review on generation, analysis, occurrence, fate and effects. Water Res 139:83–100. https://doi.org/10.1016/j.watres.2018.03.051
Wang F, Wong CS, Chen D et al (2018) Interaction of toxic chemicals with microplastics: a Critical review. Water Res 139:208–219. https://doi.org/10.1016/j.watres.2018.04.003
Wang Q, Wangjin X, Zhang Y et al (2020) The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci Total Environ 749:141603. https://doi.org/10.1016/j.scitotenv.2020.141603
Wang W, Wang J (2018) Investigation of microplastics in aquatic environments: an overview of the methods used, from field sampling to laboratory analysis. TrAC Trends Anal Chem 108:195–202. https://doi.org/10.1016/j.trac.2018.08.026
Wang X, Bolan N, Tsang DCW et al (2021) A review of microplastics aggregation in aquatic environment: influence factors, analytical methods, and environmental implications. J Hazard Mater 402:123496. https://doi.org/10.1016/j.jhazmat.2020.123496
Weber A, Jeckel N, Weil C et al (2021) Ingestion and toxicity of polystyrene microplastics in freshwater bivalves. Environ Toxicol Chem 40:2247–2260. https://doi.org/10.1002/etc.5076
Weinstein JE, Crocker BK, Gray AD (2016) From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ Toxicol Chem 35:1632–1640. https://doi.org/10.1002/etc.3432
Werder EJ, Engel LS, Richardson DB et al (2018) Environmental styrene exposure and neurologic symptoms in U.S. Gulf coast residents. Environ Int 121:480–490. https://doi.org/10.1016/j.envint.2018.09.025
Winiarska E, Jutel M, Zemelka-Wiacek M (2024) The potential impact of nano- and microplastics on human health: understanding human health risks. Environ Res 251:118535. https://doi.org/10.1016/j.envres.2024.118535
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51:6634–6647. https://doi.org/10.1021/acs.est.7b00423
Wu J, Jiang Z, Liu Y et al (2021) Microplastic contamination assessment in water and economic fishes in different trophic guilds from an urban water supply reservoir after flooding. J Environ Manag 299:113667. https://doi.org/10.1016/j.jenvman.2021.113667
Wu P, Cai Z, Jin H, Tang Y (2019a) Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci Total Environ 650:671–678. https://doi.org/10.1016/j.scitotenv.2018.09.049
Wu X, Pan J, Li M et al (2019b) Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res 165:114979. https://doi.org/10.1016/j.watres.2019.114979
Yan Z, Liu Y, Zhang T et al (2022) Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol 56:414–421. https://doi.org/10.1021/acs.est.1c03924
Yaranal NA, Subbiah S, Mohanty K (2021) Distribution and characterization of microplastics in beach sediments from Karnataka (India) coastal environments. Mar Pollut Bull 169:112550. https://doi.org/10.1016/j.marpolbul.2021.112550
Ye Y, Yu K, Zhao Y (2022) The development and application of advanced analytical methods in microplastics contamination detection: a critical review. Sci Total Environ 818:151851. https://doi.org/10.1016/j.scitotenv.2021.151851
Yee MS-L, Hii L-W, Looi CK et al (2021) Impact of microplastics and nanoplastics on human health. Nanomaterials 11:496. https://doi.org/10.3390/nano11020496
Yi X, Wang J, Li Z et al (2019) The effect of polystyrene plastics on the toxicity of triphenyltin to the marine diatom Skeletonema costatum—influence of plastic particle size. Environ Sci Pollut Res 26:25445–25451. https://doi.org/10.1007/s11356-019-05826-3
Yoon D-S, Lee Y, Park JC et al (2021) Alleviation of tributyltin-induced toxicity by diet and microplastics in the marine rotifer Brachionus koreanus. J Hazard Mater 402:123739. https://doi.org/10.1016/j.jhazmat.2020.123739
Yu X, Peng J, Wang J et al (2016) Occurrence of microplastics in the beach sand of the Chinese inner sea: the Bohai Sea. Environ Pollut 214:722–730. https://doi.org/10.1016/j.envpol.2016.04.080
Yu Y, Mo WY, Luukkonen T (2021) Adsorption behaviour and interaction of organic micropollutants with nano and microplastics – a review. Sci Total Environ 797:149140. https://doi.org/10.1016/j.scitotenv.2021.149140
Zhang J, Wang L, Trasande L, Kannan K (2021a) Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ Sci Technol Lett 8:989–994. https://doi.org/10.1021/acs.estlett.1c00559
Zhang K, Hamidian AH, Tubić A et al (2021b) Understanding plastic degradation and microplastic formation in the environment: a review. Environ Pollut 274:116554. https://doi.org/10.1016/j.envpol.2021.116554
Zhang M, Lin Y, Booth AM et al (2022) Fate, source and mass budget of sedimentary microplastics in the Bohai Sea and the Yellow Sea. Environ Pollut 294:118640. https://doi.org/10.1016/j.envpol.2021.118640
Zhang Y, Wang C, Jia R et al (2024) Transfer from ciliate to zebrafish: unveiling mechanisms and combined effects of microplastics and heavy metals. J Hazard Mater 479:135645. https://doi.org/10.1016/j.jhazmat.2024.135645
Zhang Y, Wang X, Shan J et al (2019) Hyperspectral imaging based method for rapid detection of microplastics in the intestinal tracts of fish. Environ Sci Technol 53:5151–5158. https://doi.org/10.1021/acs.est.8b07321
Zhao H-J, Xu J-K, Yan Z-H et al (2020) Microplastics enhance the developmental toxicity of synthetic phenolic antioxidants by disturbing the thyroid function and metabolism in developing zebrafish. Environ Int 140:105750. https://doi.org/10.1016/j.envint.2020.105750
Zhou Y, Yang Y, Liu G et al (2020) Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: the roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res 184:116209. https://doi.org/10.1016/j.watres.2020.116209
Zobkov M, Esiukova E (2017) Microplastics in Baltic bottom sediments: quantification procedures and first results. Mar Pollut Bull 114:724–732. https://doi.org/10.1016/j.marpolbul.2016.10.060
Download references
Open access funding provided by Stockholm University. The authors would like to acknowledge the BAPR (Baltic Phytoremediation) and Life program (grant no. LIFE15 ENV/SE/000279) projects for funding and we express our gratitude to the team at the Environmental Science laboratory of Linnaeus University for their valuable assistance during the literature review process. A. Krauklis involvement has been backed by the European Regional Development Fund through Activity 1.1.1.2, specifically the “Post-doctoral Research Aid” within Specific Aid Objective 1.1.1 of the Operational Programme “Growth and Employment” (Nr.1.1.1.2/VIAA/4/20/606, “Modelling Toolbox for Predicting Long-Term Performance of Structural Polymer Composites under Synergistic Environmental Ageing Conditions”). J. Burlakovs contribution is supported by the Marie Sklodowska-Curie COFUND project, funded under Horizon 2020 grant agreement No.847639 within the project “GeoReco.”
Author information
Authors and affiliations.
Department of Biology and Environmental Science, Linnaeus University, SE-392 31, Kalmar, Sweden
Department of Ecology Environment & Plant Sciences (DEEP), Stockholm University, Stockholm, Sweden
Department of Materials and Environmental Chemistry (MMK), Stockholm University, Stockholm, Sweden
Roshan Prabhakar
Department of Civil and Environmental Engineering, University of North Carolina at Charlotte, 9201 University City Boulevard, Charlotte, NC, 28223, USA
Visva Bharati Barua
Institute of Chemistry, University of Tartu, 14a Ravila St, Tartu, Estonia
Ivar Zekker
Riga Technical University, Riga, Latvia
Juris Burlakovs
Norwegian University of Science and Technology (NTNU), Trondheim, Norway
Andrejs Krauklis
Environmental Engineering and Recovery, Department of Biology and Environmental Science, Faculty of Health and Life Sciences, Linnaeus University, SE-392 31, Kalmar, Sweden
William Hogland
Department of Environmental Science, University of Latvia, Jeglavas Street 1, Riga, LV-1004, Latvia
Zane Vincevica-Gaile
You can also search for this author in PubMed Google Scholar
Contributions
Divya Pal: writing—review and editing, writing—original draft, validation, resources, methodology, investigation, formal analysis, data curation, conceptualization. Roshan Prabhakar: writing—review and editing, writing—original draft, validation, conceptualization. Visva Bharati Barua: writing—review and editing, writing—original draft. Ivar Zekker: writing—review and editing, writing—original draft. Juris Burlakovs: writing—review and editing, writing—original draft. Andrejs Krauklis: writing—review and editing, writing—original draft. William Hogland: project administration, writing—review and editing, writing—original draft. Zane Vincevica-Gaile: writing—review and editing figures, writing—original draft.
Corresponding author
Correspondence to Divya Pal .
Ethics declarations
Ethical approval, consent to participate, consent for publication.
All authors agree with publishing.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
(DOCX 398 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Pal, D., Prabhakar, R., Barua, V.B. et al. Microplastics in aquatic systems: A comprehensive review of its distribution, environmental interactions, and health risks. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-35741-1
Download citation
Received : 05 September 2024
Accepted : 04 December 2024
Published : 13 December 2024
DOI : https://doi.org/10.1007/s11356-024-35741-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aquatic environment
- Bioaccumulation
- Health risk
- Quantification
- Find a journal
- Publish with us
- Track your research
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected] (ME); [email protected] (LME)
Affiliation 5 Gyres Institute, Los Angeles, California, United States of America
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing
Affiliations University of California Riverside, Riverside, California, United States of America, Moore Institute for Plastic Pollution Research, Long Beach, California, United States of America
Roles Investigation, Writing – original draft, Writing – review & editing
Affiliation California State Water Resources Control Board, Sacramento, California, United States of America
Roles Conceptualization, Writing – original draft, Writing – review & editing
Affiliation Stockholm Resilience Centre, Stockholm University, Stockholm, Sweden
Roles Data curation, Writing – original draft, Writing – review & editing
Affiliations Moore Institute for Plastic Pollution Research, Long Beach, California, United States of America, Algalita Marine Research and Education, Long Beach, California, United States of America
Affiliation EOS Center, San Francisco State University, Tiburon, California, United States of America
Affiliation ABR, Inc.--Environmental Research & Services, Fairbanks, Alaska, United States of America
Roles Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing
Affiliations Facultad Ciencias del Mar, Universidad Católica del Norte (UCN), Coquimbo, Chile, Center for Ecology and Sustainable Management of Oceanic Islands (ESMOI), Coquimbo, Chile, Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Coquimbo, Chile
Affiliation Minderoo Foundation, Perth, Western Australia, Australia
- Marcus Eriksen,
- Win Cowger,
- Lisa M. Erdle,
- Scott Coffin,
- Patricia Villarrubia-Gómez,
- Charles J. Moore,
- Edward J. Carpenter,
- Robert H. Day,
- Martin Thiel,
- Chris Wilcox

- Published: March 8, 2023
- https://doi.org/10.1371/journal.pone.0281596
- Reader Comments
As global awareness, science, and policy interventions for plastic escalate, institutions around the world are seeking preventative strategies. Central to this is the need for precise global time series of plastic pollution with which we can assess whether implemented policies are effective, but at present we lack these data. To address this need, we used previously published and new data on floating ocean plastics (n = 11,777 stations) to create a global time-series that estimates the average counts and mass of small plastics in the ocean surface layer from 1979 to 2019. Today’s global abundance is estimated at approximately 82–358 trillion plastic particles weighing 1.1–4.9 million tonnes. We observed no clear detectable trend until 1990, a fluctuating but stagnant trend from then until 2005, and a rapid increase until the present. This observed acceleration of plastic densities in the world’s oceans, also reported for beaches around the globe, demands urgent international policy interventions.
Citation: Eriksen M, Cowger W, Erdle LM, Coffin S, Villarrubia-Gómez P, Moore CJ, et al. (2023) A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required. PLoS ONE 18(3): e0281596. https://doi.org/10.1371/journal.pone.0281596
Editor: Judi Hewitt, The University of Auckland - City Campus: University of Auckland, NEW ZEALAND
Received: January 12, 2022; Accepted: January 26, 2023; Published: March 8, 2023
Copyright: © 2023 Eriksen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data from the Plastic Marine Pollution Global Dataset, Modeling code, and Trend Reversal data are available open source at github.com. https://github.com/wincowgerDEV/ocean_plastic_modeling .
Funding: ME received funding from the Baum Foundation to support expeditions and sample collection ( http://thebaumfoundation.org/ ). MT was supported by the European Union’s H2020 research and innovation programme MINKE project (under Grant Agreement No 101008724). These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Understanding the occurrence and trends of plastic abundance in the world are foundational to assessing current and potential future risks to humans and ecosystems [ 1 ]. Modeling plastic pollution’s fate and transport in the ocean surface layer (OSL) is complicated by complex mechanisms of degradation, fouling, and turbulent transport [ 2 ]. Fragmentation of large plastic results in micro- and nanoplastics leaving the OSL to shoreline and seafloor compartments, where they may cause harm to organisms through ingestion [ 3 ]. While recent modeling efforts suggest rapid export of plastic pollution away from the OSL [ 4 ], inputs are likely to continue [ 5 , 6 ]. Therefore, understanding trends in regional and global plastic pollution mass and abundance is essential to evaluating and mitigating the risks.
While challenging, quantifying the global mass of plastics has previously been estimated for the OSL at 93,000 to 578,000 tonnes [ 7 – 9 ]. Spatial and temporal data gaps and variability in station-selection, sample-collection, and analysis make interpreting snapshots in time challenging and make establishing a trend even more difficult for the OSL [ 10 ]. Edelson et al. [ 11 ] suggested that the wide variability in reported inputs reveals an urgent need for improved monitoring frameworks to facilitate global governance.
A few earlier trends offer a perspective of plastic accumulation in the oceans. Archived Continuous Plankton Recorder (CPR) samples show an increasing trend of microfibers since the 1960s [ 12 ] and an increasing trend of macroplastic entanglement since the late 1950s [ 13 ]. Day and Shaw [ 14 ] reported an increase of microplastics in the North Pacific between 1976 and 1985, and Wilcox et al. [ 15 ] observed an increasing trend in the western North Atlantic from 1986 to 2015, with a rate of increase paralleling global cumulative plastic production. Although these studies suggest long-term increases, they are only from northern oceans surrounded by the most industrialized countries. In contrast, other studies have found no evidence of a rise in plastic pollution over time [see references in [ 10 ]].
Here, we evaluate a global dataset with all available historical data to provide an estimate of the temporal tendencies of plastic concentrations in the global OSL. We also offer a historic overview of international policy measures to reduce plastic inputs; based on that evaluation we call for urgent and effective solutions.
Materials and methods
Data sets: net-tow sample collection and analysis.
We compiled data on OSL plastic abundance and distribution from published literature and unpublished sources, totaling 11,777 stations used in this trend analysis ( Fig 1 ; S1 Dataset and on GitHub https://github.com/wincowgerDEV/ocean_plastic_modeling ). Data were aggregated primarily from peer-reviewed manuscripts and previously unpublished data from 5 Gyres Institute expeditions. The data were collected with multiple methods of sea-surface sampling: manta trawl [ 8 , 16 ], AVANI trawl [ 17 ], or a rectangular neuston net [ 7 , 18 ]. We filtered the data to include samples with a lower mesh size range between 53μm and 505μm. Of the 11,777 samples in this study, 0.2% of samples were collected with a 53μm mesh (n = 27), 2.5% used 200μm (n = 303), 2.5% used 500–505μm (n = 293), and 94.7% used a 320–350μm (n = 11,154). The upper range of net openings was between 0.5m and 1m ( S1 Table ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
A total of 11,777 stations were used to model a global time trend.
https://doi.org/10.1371/journal.pone.0281596.g001
Although there was some variability in the methodology for each dataset, the methods typically were as follows: with the aid of a dissecting microscope, microplastics were manually separated from natural debris after being sorted through sieves [ 19 , 20 ], then counted individually, before all microplastics from each size category were weighed together. To compute count data (in pieces km -2 ) we divided the total count of plastics collected by the surface area of water that the trawl went through. If this metric was not provided, we computed it using trawl dimensions and distance sampled as reported in the corresponding literature. If only the month and year were provided for the date, we used the 15th of the month specified as the day. Mass was estimated with a common conversion rate reported in the literature (1.36 x 10 −2 g particle -1 ) by Morét-Ferguson et al. [ 21 ].
Ocean surface-layer basins and currents
To improve estimates of OSL plastic abundance and distribution in different ocean basins, we assigned each station to one of six basins: North Atlantic, South Atlantic, North Pacific, South Pacific, Indian, and Mediterranean. The number of stations in each basin for each year is shown in Fig 2 . We used model estimates for OSL plastic concentrations from Van Sebille et al. [ 9 ] and centered and scaled them within each ocean basin as a stable time reference to correct spatial sampling biases.
Some ocean basins are overrepresented (e.g., North Atlantic and North Pacific), whereas others have very few observations at a minority of time points (e.g., Indian, South Atlantic, South Pacific, and Mediterranean).
https://doi.org/10.1371/journal.pone.0281596.g002
Wind and waves cause downward mixing of plastic particles at the OSL, decreasing the observed concentration when winds are strong [ 22 ]. This effect can be corrected by various process-based techniques when wind velocity is known [ 9 , 15 ]. We corrected all data on ocean-surface concentrations by using the ERA-Interim Project [ 23 ] wind dataset, which provides average monthly surface wind speeds at (256 height x 512 width approximately 80 km) planet-wide spatial resolution and the Kukulka et al. [ 22 ] equation. We chose to use the Kukulka et al. [ 22 ] equation from 2012 instead of the refined Kukulka and Brunner [ 24 ] equation from 2015 because we incorporated previously published datasets that utilized the earlier 2012 equation [ 22 ], and we chose to be consistent in wind-corrected data. In cases where previous published data had been corrected, we acquired raw data and utilized the Kukulka et al. equation to standardize wind-corrected concentrations. The average expected effect of the wind correction is ~2.5 times the observed concentrations [ 22 ]. The expected ocean concentrations were derived by Lagrangian modeling of surface drifters for the year 2016 with a spatial resolution of 1° [ 9 ]. The concentrations in each basin were scaled and centered so that the expected concentration values would facilitate a regression analysis.
We modeled OSL temporal trends in plastic abundance and distribution with the above datasets corrected for wind and basin over time. Although there are many examples of global and regional plastic abundance and distribution estimates at a single point in time, temporal trends proved more challenging to produce.
Here, we used a model that removed the effects of site-bias and non-detects from the observed concentrations. We corrected for non-detects by treating zeros in the data as censored observations, in which the actual observation is below the detection level (1/volume of water sampled), instead of being considered a true zero value. We estimated the likely values of the zero records with the cenros function in the NADA package [ 25 ]; this function is a regression on ordered statistics that fits a model to the observed data and their quantiles to extrapolate the left-censored values. After correcting for non-detects with the cenros function, we estimated the actual values of the zeros to include in the model ( S1 Fig ). Plotting log-transformed observed concentration and expected concentration from an oceanographic transport model revealed an ocean-basin and concentration bias (quantile–quantile (qq) plot) ( S2 Fig ). To correct the collinear effect of expected concentration, we first fit a generalized additive model (GAM) model (gamma distribution) between log-transformed observed concentration and expected concentration from the van Sebille model [ 9 ]. We fit the model (concentration = expected concentration + basin) to the observed concentrations to get the residuals. Here the predicted density has no temporal component, only a spatial one, so the residuals from this relationship give a measure of how much an observation diverges from the relative concentration across space that we would expect. We then explored support for a time trend by modeling the residuals from the model above using a smooth function on time in a generalized additive model ( S3 Fig ). This approach is similar to adding all variables to a single model but allows us to interpret more precisely the effect of time by itself without influence from the other variables due to collinearity. We then estimated the average concentration change through time by multiplying the mean concentration observed by the reverse log-transformed residual fit. This approach allowed us to predict global plastic quantity weighted by observed mean plastic concentrations without influence from spatial effects.
Modeled results of floating microplastic item count (trillion of particles) and mass (millions of tonnes) globally for each year were calculated by averaging daily model results. Daily model results for particle counts were calculated by multiplying model results (particles km -2 ) x 361,900,000 km 2 (ocean surface-area). Daily model results for mass were calculated by multiplying 1.36 x 10 −2 g (average particle mass and weight from [ 21 ] x the number of particles.
After accounting for wind, site selection, and biases due to under-sampling, a significant trend was observed with ocean plastic through time ( Fig 3 ). Plastic concentrations in the OSL varied over time, with a dramatic increase soon after the turn of the century. There are few samples before 1990, which is reflected in the width of the confidence intervals for the left-hand side of the temporal trend; however, during the last decade of the previous century the number of samples increased, resulting in reliable trends between 1990 and 2015 ( Fig 3 ).
Change in the abundance of ocean plastic through time in trillions of particles or tens of thousands of metric tonnes using a smoothing spline and a generalized additive model. Central line is the model fit; confidence intervals are 2 times the standard error of the model.
https://doi.org/10.1371/journal.pone.0281596.g003
For the period with extensive sample coverage (1990–2015), there is substantial variability until 2004, which could be interpreted as stagnation or a decreasing trend; however, from 2005 onward, there is a consistent and rapid increase in plastic abundance ( Fig 3 ). Based on our model results, we estimate that 82–358 trillion plastic particles (mean = 171 trillion plastic particles, primarily microplastics, weighing 1.1–4.9 million tonnes (mean = 2.3 million tonnes) were afloat in 2019 ( Table 1 ).
https://doi.org/10.1371/journal.pone.0281596.t001
Previous estimates of total ocean plastic concentrations distributed the model estimates back to ocean model grids [ 7 – 9 ]. The current estimate uses an oceanographic model to estimate concentrations by location, allowing us to extract a time trend without having to build a full spatio-temporal model. Then the model mean concentration prediction multiplied by the size of the ocean is used to predict the global ocean abundance; extrapolating the model predictions to ocean basins with few samples would have incorrectly estimated our certainty in the model grids.
Our study shows a significant increase since the turn of the century for the global ocean abundance and distribution of plastics in the OSL. The wide confidence intervals from 1979 to 1990 leave no clear detectable trend. From 1990 to 2005, concentrations of plastic fluctuate during this trendless period, followed by a dramatic increasing trend from 2006 onward. These observations may have been influenced by policy interventions, plastic production, fragmentation of existing floating plastic, and/or waste management and trade.
Temporal trend
The stagnating trend observed prior to 2006 may have benefitted from important policy measures that were implemented during and just before that period ( Fig 4 ). In 1988, MARPOL added Annex V, which established legally-binding agreements among 154 countries to end the discharge of plastics from naval, fishing, and shipping fleets. These interventions were preceded by the United Nations Convention on the Law of the Sea in 1982 and the Convention on the Prevention of Marine Plastic by Dumping of Wastes and other Matter in 1972 [ 26 ]. In 1991, the Plastic Industry Trade Association launched “Operation Clean Sweep” with a goal of zero loss of plastic pellets, powders, and flakes from factories [ 26 ], with decreasing pellet ingestion in biota observed [ 27 ]; however, these and others were voluntary agreements.
A cluster of binding international policy and maritime-law interventions preceding the millennium may have played a role in slowing the increasing trend of plastic waste in the OSL.
https://doi.org/10.1371/journal.pone.0281596.g004
The rapid increase from 2005 onwards may reflect exponential growth of plastic production as it relates to inputs [ 15 ] or changes in terrestrial waste generation and management [ 28 ]. Older macroplastics adrift or stranded on shorelines or in rivers [ 4 ] continue to degrade and fragment and contribute to increases in the abundance of microplastics [ 15 ].
Despite the observed trends, there is uncertainty in collating data at a global scale. However, the dramatic increase observed herein for oceanic microplastics after 2005 parallels trends estimated for global beaches [ 29 ], which are based on completely independent observations. These parallel trends strongly suggest that plastic pollution in the world’s oceans during the past 15 years has reached unprecedented levels. Considerable data exist for the North Pacific and North Atlantic basins, although we lack information for the other ocean basins. More samples are needed in the South Atlantic, South Pacific, Mediterranean, and Indian oceans, all of which remain data-poor regions (see also [ 29 ]). In the future, if greater attention is given to the southern hemisphere, and sampling occurs at more-even intervals, models can provide outputs with higher resolution that can better identify spatial and temporal trends.
The globalization of raw plastic materials accelerated at the turn of the century [ 30 ] leading to rapid increases in import, export, and domestic production of plastic products and packaging and to increases in the amount of plastic waste generated. Simultaneously, plastic recycling, even in countries with highly developed waste-management infrastructure, has historically been low [ 6 ]. This lack of recycling has resulted in a flood of plastic products and packaging with dead-end material flows, largely due to the international trade of low-value waste plastics that remain as mismanaged waste in the receiving country [ 31 ]. The turn of the century also marked increased emissions of plastic waste from large fishing fleets and artisanal fisheries [ 32 ].
Increased international economic activity and the fragmentation and resuspension of degraded macroplastics probably drive the observed increasing trend. These factors have overwhelmed both the natural export mechanisms that transport plastic out of the OSL and any positive impact of those early binding interventions that may have driven the observed decrease toward the end of last century.
Limitations
The study results are limited by sampling distribution and the unequal distribution of observations ( Fig 2 and S4 Fig ). The vast majority of samples was collected in the North Pacific and North Atlantic Oceans ( Fig 2 ). Therefore, our results will be biased to observed trends in those ocean basins. The coefficient of variation ( S4 Fig ) had a wide spread (min = 0.447, max = 10.558, mean = 2.602), which underscores the challenge in modeling time-trends of ocean plastic concentrations accurately. The South Atlantic, Mediterranean, and Indian oceans had the lowest coefficient of variations, but that may be more due to the small number of samples and limited spatial distributions there than having smaller spatial variations.
In addition, we recognize limitations in our estimates of particle counts and mass. Particle counts may vary considerably when considering the lower size limit for particles. In terms of mass, and while we used a simple conversion of count to mass [ 12 ], new techniques are being developed (e.g., machine learning) to estimate mas and count conversions more accurately [ 33 ].
Policy implications
The accelerating abundance of plastic in the OSL demands urgent international policy intervention to minimize ecological, social, and economic harm [ 5 ]. Without substantial widespread policy changes, the rate at which plastics enter aquatic environments will increase approximately 2.6-fold from 2016 to 2040 [ 6 ].
Existing international policies on plastic are fragmented, favor business-oriented solutions [ 34 ], lack specificity, and do not include measurable targets [ 35 ], with the exception of the 2019 Plastic Waste Amendments to the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal [ 36 ]. Although legally binding, this Convention regulates plastic trade only at the very end of its life cycle, once it becomes waste. International policy interventions after 2005 generally lack robust monitoring frameworks and enforcement mechanisms [ 34 – 37 ]. Because they also are non-binding and voluntary, they are not stemming the tide [ 38 ].
For example, the New Global Economy Commitment [ 39 ] promotes a set of circular economic principles to ensure the economic viability and meaningful reduction in the production of plastic waste: restrictions on certain products, extended producer responsibility for new and existing products, and waste-reduction targets. They invite stakeholders voluntarily to adopt the goal of making 100% of plastic packaging reusable, recyclable, or compostable by 2025, yet these measures advocate for private-sector self-regulation [ 34 ]. Economic barriers to recycled plastic continue to place virgin plastics at a lower cost, as the industries that produce and use plastics resist binding commitments to use recycled plastic and design standards for efficient recyclability, leaving recycling overall to fail expectations [ 40 ]. This increased production of virgin plastics will increasingly undermine circular economic principles, including the reuse economy and global policy interventions intended to reduce the most polluting plastic products and packaging [ 41 ].
Conclusions
Although scientists continue to understand better the environmental fate of plastic pollution, there is consensus that global increases in plastic production result in dramatic increases in plastic pollution as shown herein, underscoring the urgent need for effective global governance. Currently, we are at a turning point in history. Early in 2022, at the United Nations Environmental Assembly 5.2. in Nairobi, all Member States adopted a resolution to end plastic pollution, committing to establish a legally binding global agreement that addresses the full life-cycle of plastic, including its production, design, and disposal, by 2024 [ 42 ]. The final outcome of this agreement will be a treaty, but its strength will depend on commitments by the member states and on whether measures are focused on the full life cycle of plastics, from extraction and manufacturing to its end of life [ 43 ]. Environmental recovery of plastic has limited merit, so solution strategies must address those systems that restrict emissions of plastic pollution in the first place. Therefore, establishing standardized monitoring frameworks to track global trends and creating binding and enforceable international agreements to prevent the emissions of plastic pollution are the best long-term global solutions.
Supporting information
S1 dataset. these data are available at figshare.com..
Plastic Marine Pollution Global Dataset, Modeling code, and Trend Reversal data are available open source On GitHub. The following dataset includes all data points used in our model: https://github.com/wincowgerDEV/ocean_plastic_modeling .
https://doi.org/10.1371/journal.pone.0281596.s001
S1 Fig. Correcting for non-detects.
Correcting for non-detects using the cenros function, we estimated the actual values of the zeros to include in the model (A) observed concentration histogram log+1 transformed. Shows that there is a large gap between the zero observations and the nonzeros which indicates a need to correct non-detects. (B) corrected concentrations are normally distributed in log space.
https://doi.org/10.1371/journal.pone.0281596.s002
S2 Fig. QQ plots.
(A) QQ plots for the initial model fit and (B) the residual model fit.
https://doi.org/10.1371/journal.pone.0281596.s003
S3 Fig. Global trend based on residuals.
Annual global estimates of floating microplastic particles based on residuals from 1979 to 2019 show a period slow decreasing abundance and a steady increase from 2005 onward. Residuals are in log transformed space. The shaded grey area reflects confidence intervals. The “rugplot” at the bottom of the figure provides a line for each sample to show the density of stations. The low number of stations on either end of the figure results in wide confidence intervals.
https://doi.org/10.1371/journal.pone.0281596.s004
S4 Fig. Coefficient of variation for the datasets for each year.
Sampling from year to year and basin to basin has a similar about of relative variability, which is a good thing in terms of fitting models to it.
https://doi.org/10.1371/journal.pone.0281596.s005
S1 Table. Summary of datasets used in model.
The datasets used in the model (126 total). The year (of sample collection), data source, mesh size of net, the ocean basin, and the number of observations in that dataset are summarized.
https://doi.org/10.1371/journal.pone.0281596.s006
Acknowledgments
We thank Ocean Missions for data from Iceland and Greenland and thank Ocean Research Project for data from across the Arctic. We also thank the anonymous reviewers for their highly constructive suggestions, which helped to improve this manuscript.
- View Article
- PubMed/NCBI
- Google Scholar
- 35. Karasik R, Vegh T, Diana Z, Bering J, Caldas J, Pickle A, et al. 20 Years of Government Responses to the Global Plastic Pollution Problem. Durham, NC: Duke University.; 2020 p. 311.
- 36. UN Environment Programme, Basel Convention. BC-14/12: Amendments to Annexes II, VIII and IX to the Basel Convention. UN Environment Programme, Basel Convention; http://www.basel.int/Implementation/Plasticwaste/Decisions/tabid/6069/Default.aspx
- 39. WWF, the Ellen MacArthur Foundation, BCG. The business case for a UN treaty on plastic pollution. 2020. https://www.plasticpollutiontreaty.org/UN_treaty_plastic_poll_report.pdf
- 40. Gourmelon G. Global Plastic Production Rises, Recycling Lags. Vital Signs, Worldwatch Institute; 2015.
- 41. Charles D, Kimman L, Saran N. The Plastic Waste Makers Index. Minderoo Foundation; 2021. https://www.minderoo.org/plastic-waste-makers-index/
- 42. UNEP/EA.5/L.23/Rev.1. UNEP/EA.5/L.23/Rev.1. End plastic pollution: Towards an international legally binding instrument (Draft Resolution). UNEP; 2022.

COMMENTS
Abstract Plastics have been instrumental in providing access to clean drinking water, medical applications, and improved hygiene and food safety. However, plastics also cause problems. More than 10 million tons of plastic enter the oceans annually. Marine plastic pollution has documented impacts on marine organisms and ecosystem services. The use of chemical additives in plastics also poses a ...
Plastic pollution is ubiquitous throughout the marine environment, yet estimates of the global abundance and weight of floating plastics have lacked data, particularly from the Southern Hemisphere and remote regions. Here we report an estimate of the total number of plastic particles and their weight floating in the world's oceans from 24 expeditions (2007-2013) across all five sub-tropical ...
Abstract: Plastic pollution in the ocean is a global concern; concentrations reach 580,000 pieces/km2 and production is increasing exponentially. Plastic pollution is a widespread problem ...
Development of proposals/solutions on key research gaps can open a novel pathway to address this environmental issue in an effective scientific manner. In conclusion, this paper demonstrates the current status of plastic pollution in the marine ecosystem to make aware people of a plastic-free, healthy blue ocean in the near future.
There are substantial and increasing quantities of plastic pollution in the marine environment, hereafter referred to as 'marine plastic' (Geyer et al., 2017).An estimated 4.8-12.7 million metric tons of plastic entered the world's oceans from land-based sources in 2010 alone, and the flux of plastics to the oceans is predicted to increase by an order of magnitude within the next decade ...
After being for a long time disregarded, marine plastic pollution is now a growing topic among scholars, industry and government. It represents an enormous pressing threat to the integrity of the marine ecosystem, influencing its ability to provide socio-economic benefits on which human well-being is based [1].The need to react is clear: the annual discharge of plastic into the ocean is ...
In conclusion, this paper demonstrates the current status of plastic pollution in the marine ecosystem to make aware people of a plastic-free, healthy blue ocean in the near future.
Abstract Microplastics (MPs) have become a critical pollutant, accumulating in aquatic ecosystems and posing significant environmental and human health risks. Approximately 5.25 trillion plastic particles float in global oceans, releasing up to 23,600 metric tonnes of dissolved organic carbon annually, which disrupts microbial dynamics. MPs arise from the breakdown of larger plastics, degraded ...
Therefore, plastic production has caused lots of problems for the natural environment and wildlife, especially the ocean and marine organisms. In this paper, the severity of plastic pollution and ...
As global awareness, science, and policy interventions for plastic escalate, institutions around the world are seeking preventative strategies. Central to this is the need for precise global time series of plastic pollution with which we can assess whether implemented policies are effective, but at present we lack these data. To address this need, we used previously published and new data on ...