The Berg Lab
University of washington, genome sciences.

An introduction to fruit flies
This guide is adapted from the University of Arizona Department of Biochemistry and Molecular Biophysics General Biology Program for Science Teachers: Drosophila Melanogaster and Mendelian Genetics, by Pete Geiger.
An Introduction to Drosophila melanogaster
Drosophila melanogaster is a small, common fly found near unripe and rotted fruit. It has been in use for over a century to study genetics and behavior. Thomas Hunt Morgan was the preeminent biologist studying Drosophila early in the 1900’s. He was the first to discover sex-linkage and genetic recombination, which placed the small fly in the forefront of genetic research. Due to it’s small size, ease of culture and short generation time, geneticists have been using Drosophila ever since.
Fruit flies are easily obtained from the wild and many biological science companies carry a variety of different mutations. In addition these companies sell any equipment needed to culture the flies. Costs are relatively low and most equipment can be used year after year. There are a variety of laboratory exercises one could purchase, although the necessity to do so is questionable.
Why use Drosophila?
Teachers should use fruit flies for high school genetic studies for several reasons: 1. They are small and easily handled. 2. They can be easily anesthetized and manipulated individually with unsophisticated equipment. 3. They are sexually dimorphic (males and females are different), making it is quite easy to differentiate the sexes. 4. Virgins fruit flies are physically distinctive from mature adults, making it easy to obtain virgin males and females for genetic crosses. 5. Flies have a short generation time (10-12 days) and do well at room temperature. 6. The care and culture of fruit flies requires little equipment, is low in cost and uses little space even for large cultures.
By using Drosophila, students will: 1. Understand Mendelian genetics and inheritance of traits 2. Draw conclusions of heredity patterns from data obtained 3. Construct traps to catch wild populations of D. melanogaster 4. Gain an understanding of the life cycle of D. melanogaster , an insect which exhibits complete metamorphosis 5. Construct crosses of caught and known wild- type and mutated flies 6. Learn techniques to manipulate flies, sex them, and keep concise journal notes 7. Learn culturing techniques to keep the flies healthy 8. Realize many science experiments cannot be conducted and concluded within one or two lab sessions
National standards covered in these lessons: Content: 1. Organisms require a set of instructions for specifying traits (heredity) 2. Hereditary information is located in genes. 3. Combinations of traits can describe the characteristics of an organism.
Students goals: 1. Identify questions and concepts that guide scientific investigations 2. Design and conduct scientific investigations 3. Formulate and revise scientific explanations and models using logic and evidence 4. Communicate and defend a scientific argument
The genetics of Drosophila are well documented and several public-domain web sites feature the complete annotated genome . Therefore, those teachers or students wishing to see where their mutations occur have a ready reference available.
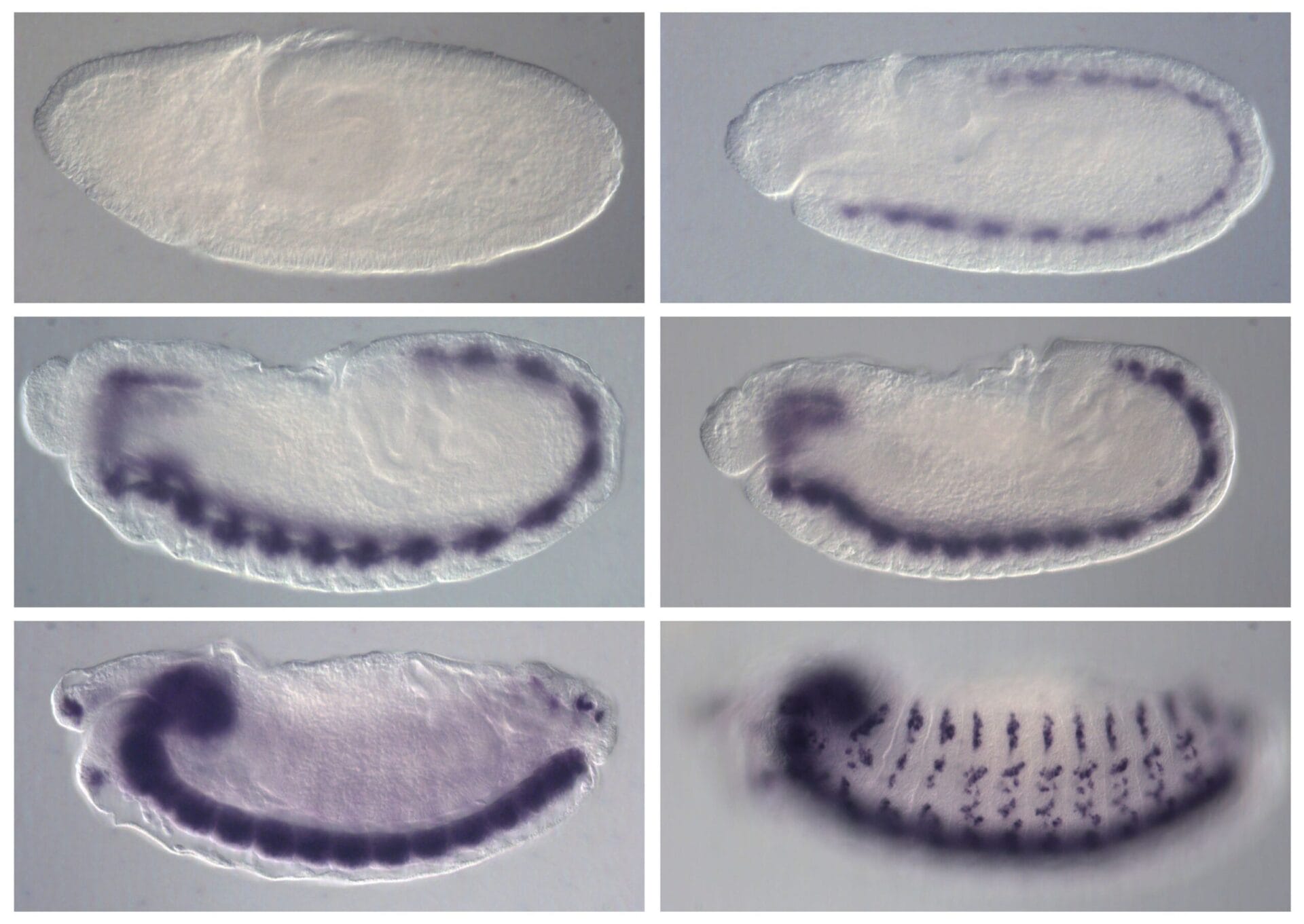
Since Drosophila has been so widely used in genetics, there are many different types of mutations available for purchase. In addition, the attentive student may find mutations within their own wild-caught cultures since, due to a short generation time, mutations are relatively common compared to other animal species. Classification Domain: Eukarya Kingdom: Animalia Phylum: Arthropoda Class: Insecta Order: Diptera Family: Drosophilidae Genus: Drosophila (“dew lover”) Species: melanogaster (“dark gut”) Life cycle of Drosophila melanogaster Drosophila melanogaster exhibits complete metamorphism, meaning the life cycle includes an egg, larval (worm-like) form, pupa and finally emergence (eclosure) as a flying adult. This is the same as the well-known metamorphosis of butterflies. The larval stage has three instars, or molts.
• The generation time of Drosophila melanogaster varies with temperature. The above cycle is for a temperature of about 22°C (72°F). Flies raised at lower temperature (to 18°C, or 64°F) will take about twice as long to develop. • Females can lay up to 100 eggs/day. • Virgin females are able to lay eggs; however they will be sterile and few in number.
After the eggs hatch, small larvae should be visible in the growing medium. If your media is white, look for the small black area (the mouth hooks) at the head of the larvae. Some dried premixed media is blue to help identify larvae however this is not a necessity and with a little patience and practice, larvae are easily seen. In addition, as the larvae feed they disrupt the smooth surface of the media and so by looking only at the surface one can tell if larvae are present. However, it is always a good idea to double check using a stereo microscope. After the third instar, larvae will begin to migrate up the culture vial in order to pupate.
Care, Maintenance and Manipulation of Drosophila
Introduction In order to incorporate fruit flies in the classroom, it will be necessary to maintain cultures of flies for manipulation in crosses and as a backup for any mishaps which may occur. Culturing is very easy and it is recommended to have students maintain their own cultures of flies. In that way, each student or group would be directly responsible for the care and long-term maintenance of the flies, including making large culture populations for their crosses. When directly involved, students gain proficiency and a greater understanding of the flies requirements and behavior. The teacher should remain as coach, not lecturer, assisting students in techniques. The instructor needs to maintain stock cultures of all strains and mutants used by students in case the aforementioned unforeseeable incident occurs and student cultures die out or become intermixed. Losing cultures is the exception rather than the rule, and as long as students re-culture their flies on a regular basis and no mass contamination occurs, flies can be maintained for decades. Bottles and vials Thomas Hunt Morgan used glass milk bottles for his experiments and, indeed, any container will do, including baby jars and assorted containers. However, for ease of culturing and transferring cultures, uniform bottles and vials are the best approach. Both can be purchased from a biological supply store. Bottles are used mainly for the maintenance of large populations of flies whereas culture vials are useful for maintaining smaller populations and are the preferred container for constructing student crosses. If there is a desire to maintain stock cultures for a long period of time, or to reuse bottles and vials it is important completely clean and sterilize them. This is to prevent outbreaks of pests and diseases.
To clean bottle and vials, first freeze them to kill any flies in them. Remove the food, wash well, then sterilize by autoclaving (for 20 minutes at 121°C and 15 psi; if containers are plastic, be sure they can be autoclaved) or washing in a 10% chlorine bleach solution.
Bottles and vials can be purchased in a variety of sizes and materials. Glass is effective, however if dropped a student could lose 2 weeks of data in a single spill. Autoclaved (sterile) plastic vials are available and are preferable for student use. Vial sizes range from 96 mm by 25 mm to larger sizes, however the smaller size is recommended for making crosses and maintaining small cultures. There are a variety of plugs available from soft cotton to foam plugs. This is a matter of preference and costs, however cotton works fine and can be bought at a local drug store in a pinch. Where to buy supplies: Carolina Biological Supply Company FlyStuff.com , A division of Genesee Scientific What they look like:
Fly food The first step in preparing culture vials is adding food media. There are a variety of types of food available for the flies; some require cooking and others are bought already prepared and dehydrated. The latter can be purchased from a biological supply company. This is, of course, much quicker and easier than preparing cooked media, so much so that students can fill their own vials with media. However, it must be completely rehydrated for best results, since this is the only water source for adults and larvae. Therefore, follow the suggestions below to ensure a completely hydrated media:
Dehydrated media Add dry media to the bottle or vial to about 1/5 to 2/5 volume. Add water until media appears completely moistened. Allow the vial to sit for a few minutes, adding additional water if necessary until the media is completely hydrated. The surface should be moist with a shiny appearance and there should be no spaces in the media. If the media is not completed hydrated, production of vigorous cultures is compromised. Flies may be added minutes after media has been hydrated. Remember to add several grains (but not more) of yeast to the media surface before adding flies.
Cooked media When dispensing cooked media, it should fill the culture vial, bottle or vial 1/5th to 2/5th full. Keep the media out overnight to cure, keeping the vials covered with cloth to keep wild flies from laying eggs in them. The next day, add yeast and plugs. Refrigerate any unused media vials. Cooked media can be stored in a refrigerator for several weeks. Allow media to warm to room temperature before adding flies. Do not allow media to dry out. Environment The easiest way to grow flies is at room temperature. However, the optimum rearing condition is a temperature of 25°C and 60% humidity. In these conditions generation time is shorter (9-10 days from egg to adult). Unless equipment is readily available this is unnecessary for successful rearing and crossing of flies. It is preferable to keep flies out of drafts and direct sunlight or heat sources. These will rapidly dry the media, necessitating frequent media changes and the potential to dehydrate the flies. Anesthetizing flies The problem with fruit flies is that they fly! Therefore a variety of methods have been developed to anesthetize flies. Include are ether, commercial brands such as Flynap, carbon dioxide, and cooling. Each has its strengths and weaknesses. Ether is flammable, has a strong odor and will kill flies if they are over-etherized (and can anesthetize younger students!). Flynap, from Carolina Biological, is messy and has an odor that some find offensive. Each of these, however, requires low-cost equipment which can be easily purchased. Carbon dioxide works very well, keeping flies immobile for long periods of time with no side effects, however CO 2 mats (blocks) are expensive and a CO 2 source (usually a bottle) and delivery system (vials and clamps) are necessary, increasing the costs. If resourceful, one can use the CO 2 emitted from Alka-Seltzer tablets to anesthetize flies for short periods of time. Set up a large test tube with a tube and stopper system. Add water in the tube, then the Alka-Seltzer tablet. Carbon dioxide gas will be emitted.
The least harmful to the flies is either carbon dioxide or cooling anesthetizing. Of these two choices, cooling is the simplest, requiring only a freezer, ice and petri dishes. In addition, it is the only method which will not affect fly neurology, therefore behavior studies may begin after the flies have warmed up sufficiently. Anesthetizing flies by cooling In order to incapacitate the flies, place the culture vial in the freezer until the flies are not moving, generally 8-12 minutes. Dump the flies onto a chilled surface. This can be constructed by using the top of a petri dish, adding crushed ice, then placing the bottom of the petri dish on top. Adding flies to this system will keep them chilled long enough to do each experiment. Simply place the flies back into the culture vial when finished. Flies will “wake up” relatively quickly once off the ice, so keep them cold. There are no long-lasting side effects to this method, although flies left in the refrigerator too long may not recover. Another way to keep flies chilled is adding water to zip-lock type freezer bags, place in the freezer with a petri dish nestled on the bag, and allow to freeze. Transferring flies from one vial to another Flies should be transferred every 10 to 14 days. Students should maintain a backup culture of their flies and the instructor should maintain backup stock cultures of all fly strains. There are two basic ways to transfer flies when forming new cultures. One requires no anesthetizing but quick hands. A) Place a funnel in the mouth of a fresh culture vial that already has media added. In the old vial (the one with flies in it), gently tap the flies down by softly tamping the vial on a soft surface, such as a mouse pad. The flies will fall to the bottom and remain there for a few seconds (no more than that!), enough time to quickly take the plug off the vial, invert it into the funnel, and gently tamp, together, the two vials to force flies down into the new vial. B) An alternative way is to put the flies in the freezer for about 8 minutes. This will cause the flies to fall into a state of stupor. After placing a funnel on the new vial, invert the vial with motionless flies into the funnel. This is not as much fun but you won’t have any flies flying around the classroom. Sexing flies It is quite easy to tell males from females and with a little practice students will become confident of their ability to do so. Notice that males are generally smaller and have a darker and more rounded abdomen. The coloration of the abdomen is the easiest to recognize. In addition, males have tarsal sex combs on their first pair of legs. These are black and very distinctive but can only be seen under relatively high magnification. With a little practice, by looking at the abdomen students will become proficient in accurately sexing flies. Sexing flies is critical when making crosses, so be sure student are confident in identifying the difference between the sexes. In order for students to feel comfortable sexing flies, give or have them obtain 25 or more mixed sex flies and allow them to sort the flies into two piles, male and female. Other students in the group and the instructor should verify the sorting. Each member of the group should be able to sex flies. Pictures of males and females
Note the darker abdomen and more rounded appearance of the male. Females also tend to be larger. Collecting virgin females While it’s a simple matter of placing virgin females with males, it is important to recognize the time factor involved for obtaining virgins. Females remain virgins for only 8-10 hours after eclosure and must be collected within this time frame. NOTE: Females have the ability to store sperm after a single mating, so if the female for a cross is not a virgin, you will not know the genotype of the male used for your cross. It is strongly suggested that you obtain extra virgins in case a mistake is made in identification or the fly dies before mating and egg lying can occur. In a strong culture, multiple virgin females should be easily obtained. Although females are able to lay eggs as virgins, they will be sterile and no larvae will be produced. Below are three ways to obtain virgins, the ‘removal method’ being most encouraged for beginners. Removal method Remove all flies 8-10 hours before collecting (generally this is done first thing in the morning). Visually inspect surface of food to ensure complete removal of flies. After 8-10 hours (usually before you leave work) collect all females that are present. All will be virgins. Place in a fresh culture vial and wait 2-3 days look for larvae. Virgin females can lay eggs, but they will be sterile. Since they are photoperiod- sensitive, females tend to eclose early in the morning. Therefore early collections will ensure the greatest number of virgins for experimentation. However, collection is possible later in the day. Visual method Being able to recognize virgin females removes the necessity of emptying culture vials on a timely basis and allows students to collect their own without the necessity of coming to class at odd times of the day. Note that virgin females are much larger than older females and do not have the dark coloration of mature females. In addition, in the early hours after eclosure, there will be visible a dark greenish spot (the meconium, the remains of their last meal before pupating) on the underside of the abdomen. Temperature cycling It is possible to maximize the number of virgins in a morning collection by using temperature cycling. When cultures are maintained at a temperature of 18°C, development is slowed so females will not mate until 16 hours after enclosure. By removing flies in the afternoon/evening and placing the vials in an 18°C incubator, 98% of flies obtained in the morning will be virgins. Placing virgins in their own vials for 2-3 days will eliminate those 2% that are non-virgins. Pictures of virgin males and females:
Crossing flies Once females are deemed virgins, add males. When setting up crosses, a 3:1 ratio of virgin females to males is ideal. Generally, males will mate more efficiently if they have matured 3 days or longer. Be sure to select robust, healthy males; the older the flies, the lower the mating efficiency. Mating occurs quickly and the behavior is interesting to watch, but will not be addressed here. Females begin laying fertile eggs soon after mating. Refer to the life cycle chart for evidence of F1 larvae. Remove adults once it has been established that enough larvae are present (typically 7-8 days after the cross) since you may not be able to distinguish parents from the F1 generation. Killing Flies: The Morgue This is an unfortunate necessity when using flies. A bottle or beaker with soapy water, or mineral oil is generally used. Dump anesthetized flies directly into the soapy water or mineral oil where they drown. A bottle (beaker, or screw-capped jar) filled with ethanol or isopropanol can also be used as a morgue. Basic Drosophila Genetics Nomenclature and Definitions
Drosophila melanogaster flies have 4 chromosomes. The genotype is written as:
Chromosome Chromosome or Chromosome / Chromosome
This common nomenclature shows one chromosome on top and its homologue on the bottom, as the chromosomes would appear during meiosis when contributing gametes.
When writing the genotype, in general, chromosomes are separated with a semicolon.
X chromosome; chromosome II; chromosome III; chromosome IV
Wild-type is denoted as “+” or WT
Dominant mutations are written with a capital letter: For example: Bar or B
Recessive mutations are written with a lower case letter: For example: white or w
Mutations are alleles (alternative forms of a gene occupying a given locus on a chromosome) that are inherited with chromosomes.
Homozygote – An individual with the same allele at corresponding loci on the homologous chromosomes.
Heterozygote – An individual with different alleles at corresponding loci on the homologous chromosomes.
Genotype – The genes that an organism possesses.
Phenotype – The observable attributes of an organism.
P1 – Parental generation.
F1 – Filial generation, or offspring generation. F1 is the first offspring generation.
F2 – The second offspring generation. Other great web resources:
Gerard Manning wrote a simple introduction to Drosophila genetics .
Genetics on the Fly: A Primer on the Drosophila Model System by Karen G. Hales et al (2015).
Taking Stock of the Drosophila Research Ecosystem by David Bilder and Kenneth D. Irvine (2017).
FlyBase is an encyclopedic resource for Drosophila researchers, with detailed information on fly stocks, genes, mutants, researchers, publications and much more.
“Sex Limited Inheritance in Drosophila” (1910), by Thomas Hunt Morgan
In 1910, Thomas Hunt Morgan performed an experiment at Columbia University, in New York City, New York, that helped identify the role chromosomes play in heredity. That year, Morgan was breeding Drosophila , or fruit flies. After observing thousands of fruit fly offspring with red eyes, he obtained one that had white eyes. Morgan began breeding the white-eyed mutant fly and found that in one generation of flies, the trait was only present in males. Through more breeding analysis, Morgan found that the genetic factor controlling eye color in the flies was on the same chromosome that determined sex. That result indicated that eye color and sex were both tied to chromosomes and helped Morgan and colleagues establish that chromosomes carry the genes that allow offspring to inherit traits from their parents.
Prior to Morgan’s fly experiments, other researchers were studying heredity. In 1865, scientist Gregor Mendel in eastern Europe published an article describing heredity experiments he had performed using pea plants. By mating pea plants, Mendel observed that the resulting offspring inherited characteristics, such as seed color and seed shape, in predictable patterns. Mendel hypothesized that there were heritable factors, later called genes, controlling the development of those characteristics.
By the early 1900s, other scientists aiming to explain heredity began to reapply Mendel’s theory. In the late nineteenth century, researchers discovered structures inside the nuclei of cells. Researchers called those structures chromosomes because of the way staining materials colored them. Staining chromosomes enabled researchers to observe chromosomes throughout development. In 1902, Walter Sutton, a researcher at Columbia University, and Theodor Boveri, a researcher at the University of Würzburg in Würzburg, Germany, each observed that chromosomes behaved in a manner that was consistent with Mendel’s theories. Boveri and Sutton hypothesized that chromosomes carried heritable factors, or genetic material. Researchers called Boveri and Suttons’ theory the Boveri-Sutton chromosome theory.
By 1904, Morgan had begun to study the processes that affect heredity and development at Columbia University. However, Morgan, like other scientists at that time, was reluctant to accept the Boveri-Sutton chromosome theory. Morgan argued that scientists had a bias towards associating phenomena, like the inheritance of traits, with known structures, like the chromosome. Similarly, he argued that if one gene didn’t explain a phenomenon, scientists could argue that any number of genes might. In 1910, Morgan published an article explaining why he was reluctant to accept the Bover-Sutton chromosome theory.
Later that year, Morgan made an observation that eventually provided evidence in support of the chromosome theory. In 1910, Morgan was studying Drosophila at Columbia University to find what he called mutants, or individual flies that had atypical, heritable characteristics, such as white eyes instead of the normal red eyes. In May of 1910, after breeding thousands of flies, he observed a single male fly with white eyes, which he called a white mutant. Typically, both male and female flies have red eyes. To explain the white eye mutation, Morgan bred the mutant fly and observed how the mutation was inherited throughout successive generations.
In 1910, Morgan published details of his research in an article titled “Sex Limited Inheritance in Drosophila." First, Morgan took the white mutant and bred it with pure red-eyed female flies. All of the females that resulted from that breeding had red eyes. Morgan then took those red-eyed females and mated them with the original white-eyed mutant male to determine whether or not the inheritance of eye color followed Mendel’s inheritance patterns. If Mendel’s patterns applied to Morgan’s flies, there would be one white-eyed fly to every three red-eyed flies in the resulting generation of flies, regardless of sex. Although Morgan did observe one white-eyed fly to every three red flies, that inheritance pattern was not shared equally across males and females. Most of the white-eyed flies were male. That result indicated that the flies did not follow Mendel’s ratio in a traditional sense.
After observing the white-eye inheritance pattern, Morgan hypothesized that a factor, or gene, controlling eye color was located on the X chromosome. Female flies have two X chromosomes, and males have one X chromosome and one Y chromosome. If a trait, like eye color, correlated with a specific factor on the X chromosome, then the trait was called X-linked. Because males only have one X chromosome, they display all X-linked traits. Females, on the other hand, often need an X-linked trait to exist on both X chromosomes to display that trait. Morgan hypothesized that, in his breeding experiment, the first generation of flies contained males only with white eyes because the gene controlling eye color was on the X chromosome. Males displayed the white eye trait because the trait was present on their only X chromosome. Females did not display the white eye trait because the trait was only present on one of their X chromosomes.
To test his hypothesis that the white-eyed trait was on the X chromosome, Morgan mated other specific groups of flies together and observed the offspring. Prior to doing so, Morgan predicted what the sex and eye color ratios of the offspring would be if his hypothesis were true. By comparing the observed results with the predicted results, Morgan determined that his hypothesis was supported. In one mating, Morgan took a red-eyed male and mated it with a white-eyed female. He predicted and observed that half of the flies would be red-eyed females and the other half would be white-eyed males. That mating showed that the occurrence of the white-eyed trait is limited to the X chromosome, as only male offspring were capable of displaying the white-eyed trait with a single copy of the trait. Morgan showed that inheritance of a trait could differ between sexes.
In the following years, Morgan and a group of scientists at Columbia University established the chromosome theory of inheritance, which described the role that chromosomes play in heredity. In 1911, Morgan published more details of his experiments with the white-eyed mutant, an account in which Morgan explicitly stated that chromosomes carry heritable factors, or genes. In 1915, Morgan, and his colleagues, Alfred Henry Sturtevant, Calvin Bridges, and Herman Joseph Muller published the book Mechanism of Mendelian Heredity . That book contained contemporary scientific information about heredity and included the results of Morgan’s white-eyed mutant experiments.
In 1933, Morgan won the Nobel Prize in Physiology or Medicine for his work establishing the chromosome’s involvement in heredity.
- Boveri, Theodor. “Über mehrpolige Mitosen als Mittel zur Analyse des Zellkerns (On multipolar mitosis as a means to analyze the cell nucleus).” Verhandlungen der physicalisch-medizinischen Gesselschaft zu Würzburg ( Proceedings of the physical-medical company at Wurzburg ) 35 (1902): 67–90. http://publikationen.ub.uni-frankfurt.de/frontdoor/index/index/docId/15991 (Accessed April 2, 2017).
- Kandel, Eric R. “Thomas Hunt Morgan at Columbia University.” Columbia University Living Legacies. http://www.columbia.edu/cu/alumni/Magazine/Legacies/Morgan/ (Accessed March 25, 2017).
- Mendel, Gregor Johann. “Versuche über Pflanzen-Hybriden (Experiments Concerning Plant Hybrids)” [1866]. In Verhandlungen des naturforschenden Vereines in Brünn ( Proceedings of the Natural History Society of Brünn ) IV (1865): 3–47. Reprinted in Fundamenta Genetica , ed. Jaroslav Krízenecký, 15–56. Prague: Czech Academy of Sciences, 1966. http://www.mendelweb.org/Mendel.html (Accessed March 25, 2017).
- Morgan, Thomas H. "Chromosomes and heredity." The American Naturalist 44 (1910): 449–96. http://www.jstor.org/stable/pdf/2455783.pdf (Accessed March 25, 2017).
- Morgan, Thomas H. "Sex Limited Inheritance in Drosophila." Science (1910): 120–2. http://www.jstor.org/stable/pdf/1635471.pdf (Accessed March 25, 2017).
- Morgan, Thomas H. “Random Segregation Versus Coupling in Mendelian Inheritance.” Science (1911): 384. http://science.sciencemag.org/content/34/873/384 (Accessed April 2, 2017).
- Morgan, Thomas H., Alfred H. Sturtevant, Hermann J. Muller, and Calvin B. Bridges. The Mechanism of Mendelian Heredity . New York: Henry Holt and Company, 1915. http://www.biodiversitylibrary.org/bibliography/22551#/summary (Accessed March 25, 2017).
- Nobel Prizes and Laureates. “The Nobel Prize in Physiology or Medicine 1933.” The Official Web Site of the Nobel Prize. https://www.nobelprize.org/nobel_prizes/medicine/laureates/1933/ (Accessed April 2, 2017).
- Sutton, Walter S. "The chromosomes in heredity." The Biological Bulletin 4 (1903): 231–50. http://www.biolbull.org/content/4/5/231.full.pdf (Accessed March 25, 2017).
How to cite
Articles rights and graphics.
Copyright Arizona Board of Regents Licensed as Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported (CC BY-NC-SA 3.0)
Last modified
Share this page.
Why the Fly?
Geneticist stephanie mohr delves into science’s favorite winged model organism.

For more than 100 years, the fruit fly Drosophila melanogaster has played a starring role in biomedical research, revealing fundamental principles of genetics and development, illuminating human health and disease and earning scientists six Nobel prizes to date.
Why do these flies continue to attract so much scientific interest even as research tools have grown more sophisticated over the decades?
Get more HMS news here.
Harvard Medicine News asked self-described “fly person” Stephanie Mohr , lecturer on genetics at Harvard Medical School and author of the book First in Fly: Drosophila Research and Biological Discovery , to explain.
As director of the Drosophila RNAi Screening Center at HMS, Mohr helps fruit-fly researchers with cell screening, fly stock production and bioinformatics services. She has also studied fruit flies for more than 15 years.
HMN: How did you get your start as a “fly person”?
MOHR: I was an undergraduate at Wesleyan University in Middletown, Connecticut, and I had a work-study job washing dishes for the biology and biochemistry departments. I would sit there and put one tub of dishes into the autoclave and take them out and do my homework in the meantime. I wasn’t too impressed by the fly lab because it always had the same set of dishes, these identical half pint-sized bottles used to culture flies. The other labs had giant cylinders and flasks and beakers of different sizes. That seemed like a reason to judge! I was a freshman—what did I know?
Later, in graduate school, I didn’t get into the lab I was most excited about, so I was kind of left hanging. I asked an instructor if I could do an extra rotation in his lab, which was a fly lab. It felt like a last-ditch effort, but it turned out to be a perfect match intellectually. He ended up becoming my PhD advisor, and I’ve been studying flies ever since.
HMN: Why has so much research been conducted in fruit flies?
MOHR: It’s part accident and an awful lot rational. There isn’t any particular reason it had to be this fly, but the fact that Drosophila melanogaster caught on has provided a number of strategic advantages.
Compared to humans, it has a simple genome with only four pairs of chromosomes. In the early years, that was a significant advantage. Also, some fly tissues have highly polyploid chromosomes—many copies of chromosomes within a single cell—which, even with the microscopy of the early 20th century, let early geneticists see things they couldn’t see in ordinary cells. They could literally see something like an inversion in the DNA, where a piece of a chromosome has been flipped around. That was a big deal for research as well.
One of the most exciting things we’ve learned in the post-genomic era is how incredibly conserved genes and pathways and genetic activities that control organism-level phenomena are across organisms. In the early days it didn’t matter how much flies were like people, because we were learning something useful at a basic level about how inheritance works. Now we’ve realized just how many similarities there are and how those similarities can be exploited to learn about human health and disease.
And then on a practical level, Drosophila has a short life cycle of about two weeks, and females have a lot of offspring—they can lay hundreds of eggs in a few days. Both these features make them a great system for genetic experiments. They’re also easy to culture in the lab, living off a mix of corn meal, sugar and yeast, and it’s easy to tell males apart from females.
HMN: What have fruit flies taught us about genetics and biology?
MOHR: The full list would be very long! Early on, the fly was highly relevant for gaining an understanding of mechanisms of inheritance. Another area where flies have had an amazing impact is in developmental biology and signal transduction, or the ways cells receive and respond to messages. Part of that legacy remains in the names we’ve given to signaling pathways in humans, like Notch, which is named for the notched pattern that a mutation of the gene can create on fly wings. Flies taught us what the components of many signaling pathways are, how they’re wired up with one another and what acts on them positively and negatively.

A cool example of signaling specifics is planar-cell polarity. That’s kind of a mouthful, but it just refers to cells on a flat surface that have a polarity, a direction. If you look at your forearm, you see small hairs, and most are pointing in the same direction. It wouldn’t be a big deal on your arm if a few were pointing in a different direction, but in something like the inner ear, those oriented projections make it possible for us to hear. The early work in uncovering how polarity is established across a plane of cells was done in Drosophila .
Relatedly, Drosophila has had a profound effect on our understanding of normal development, where you have to tell cells to divide, then stop dividing, then take on an identity. That ends up having a tremendous impact on our understanding of cancer, where all those same kinds of things have gone wrong. There’s a direct connection, in my view, between the work in fly development and what we now know about cancer and cancer treatments that act on those pathways.
There are so many more examples of what we’ve learned from Drosophila , such as how flies helped us recognize that X-rays are potent mutagens, uncover the cellular mechanisms of innate immune responses, understand the genetics of behavior—the list goes on. I couldn’t fit all of the examples even in this book.
HMN: Obviously, fruit flies aren’t people. How useful are they for understanding human health and disease?
MOHR: There are two answers I would give. One is fundamental understanding. If you want to know what all the parts do, it’s easier to start with a simpler model. Once you identify what a gene does in one organism, which we can do in flies with relative ease, you can look for similar genes in more complex organisms and make rational hypotheses about their activity. This has proved over and over to be a useful approach.
The second answer is that flies serve as a living petri dish. I can easily grow tens of thousands of flies with the same genotype, quickly assess the effects of a mutation and learn what’s going wrong at the cellular level. This is especially useful as we explore the genetic underpinnings of human disease. Genome-wide association studies, and known and suspected cases of inherited disease, are leading to a lot of good candidates for genes that seem to influence the likelihood of getting a disease. Some will turn out to be real and some will be red herrings. Flies offer a platform to start sorting through those candidates and narrow down the true culprits.
We know now that about 60 percent of all human genes, and 75 percent of disease-associated genes, have equivalents, or orthologs, in Drosophila . Studying these genes has taught us about a whole range of disease mechanisms. One area with a lot of promise is age-related diseases, including neurodegenerative diseases. Flies live for about two or three months. That’s long enough to see age-related changes—they slow down with age in ways similar to what happens in people—but short enough to accelerate the research.
HMN: What made you decide to write this book for nonscientists?
MOHR: If we want to convince society to continue to invest in research, I think we have a responsibility to show what the research is providing. It’s important for scientists working in any system to find ways to send the message that investments in biological and biomedical research have lasting value.
I’m the only scientist in my family. Over the years, I’ve found ways to explain to them what’s interesting to me about fly research and why they might be interested too. I find myself explaining even to other colleagues in biology how and why Drosophila continues to be a relevant model. I felt a book could be useful in conveying that story more broadly.
And it seems people want to know. I hear from folks who say, “Oh, yeah, in high school I looked at red- and white-eyed flies.” They can relate to this, and they’re amazed and delighted, I think, to learn more about what people are doing with flies today and to hear some of the stories about human disease.
This interview was edited for length and clarity.
Related News

A Universal Gene Therapy for Diamond-Blackfan Anemia Is Poised for Clinical Trials
Treatment designed to work across different genetic mutations

Second Gene Therapy Shows Promise for Syndrome Involving Blindness, Deafness
Method restored hearing, showed signs of improved vision in animals and organoids

Ancient DNA Challenges Stories Told About Pompeii Victims
Genetic data contradict, enrich interpretations of archaeological site
Trending News

Personalizing the Complete Blood Count Test Could Improve Patient Care
Study suggests using patients’ own reference points for standard blood test

Why Do Gliomas Tend To Recur in the Brain?
First look at neuron-tumor connections illuminates formation, spread


A Drug-Free Nasal Spray May Shield Against Respiratory Infections
Gel-like coating wards off cold viruses, flu, and more in preclinical studies
© 2024 by The President and Fellows of Harvard College
This page has been archived and is no longer updated
Thomas Hunt Morgan: The Fruit Fly Scientist
Innovator. Thomas Hunt Morgan began his career when genetics was not a defined field of study, and biology was primarily based on observation and classification. Morgan valued experimentation over observation, and he became interested in broad questions about the very nature of life. What were inherited factors? Where were they located? How were they passed from one generation to the next? Incredibly, Morgan tackled these questions with the help of the common fruit fly.
Young naturalist. Morgan was born on September 25, 1866, in Lexington, Kentucky, the son of a former Confederate officer, and the great-grandson of Francis Scott Key, composer of the "Star-spangled Banner." His career as a naturalist began during childhood with the collection of bird eggs and fossils. He began college at age 16; by age 24, he had earned his Ph.D. from Johns Hopkins University. His research interests were biology, embryology, and marine life. In fact, Morgan would continue to spend summers at the Marine Biological Laboratory in Woods Hole, Massachusetts, for most of his life.
The fly room. Morgan wanted to understand heredity and mutation, which is genetic change. After teaching for 13 years at Bryn Mawr College, he moved on to Columbia University where he established the famous "fly room." The Drosophila melanogaster , or fruit fly, is a good genetic research subject because it can be bred cheaply and reproduces quickly. Morgan was not the first to use the fruit fly as a subject, but his innovation and success popularized its use. Simple in design and easy to conduct, his early experiments are classics in genetics. Even today, no undergraduate genetics education is complete without some time spent breeding Drosophila .
The discoveries. By painstakingly examining thousands upon thousands of flies with a microscope and a magnifying glass, Morgan and his colleagues confirmed the chromosomal theory of inheritance : that genes are located on chromosomes like beads on a string, and that some genes are linked (meaning they are on the same chromosome and always inherited together). One of his students, Alfred Sturtevant, created the first ever genetic map , a landmark event in genetics.
Moving on. In 1928, Morgan became the head of the new biology department at the California Institute of Technology. He used his position to focus new research on experimentation versus passive observation, and he was finally able to establish a lab dedicated to marine biology research, which kept him occupied for the rest of his life.
Lasting legacy. Morgan's work on the role of chromosomes in heredity was recognized in 1933 with the Nobel Prize in physiology or medicine. He continued to work until his death on December 4, 1945, at age 79. Colleagues remember him as a generous man with an infectious enthusiasm for his work. As a mentor Morgan had a knack for spotting and fostering talent, and many of his students went on to make important contributions to their fields.
Further Exploration
Key Questions
Key Concepts
Topic rooms within Genetics

Other Topic Rooms
- Gene Inheritance and Transmission
- Gene Expression and Regulation
- Nucleic Acid Structure and Function
- Chromosomes and Cytogenetics
- Evolutionary Genetics
- Population and Quantitative Genetics
- Genes and Disease
- Genetics and Society
- Cell Origins and Metabolism
- Proteins and Gene Expression
- Subcellular Compartments
- Cell Communication
- Cell Cycle and Cell Division
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
- Introduction to genomics
- In the cell
- Health and disease
- Living things
- Methods and technology
- Science in society
- Resources for 5-12 year olds
- Resources for 13-18 year olds
- Resources for educators
- Resources for discussions
- Careers in genomics
Explore Genomics Methods and Technology
Model organisms: the fruit fly
The fruit fly ( Drosophila melanogaster ) has been used in research for more than 100 years, revealing important information about genetics and development.
- Model organisms are non-human species that are used in research to help us understand specific areas of biology.
- The fruit fly ( Drosophila melanogaster, or ‘Drosophila’) is the most used and one of the most well understood model organisms.
- Scientists use Drosophila to understand more about genetics and the way animals develop and grow.
The fruit fly ( Drosophila melanogaster )
- The fruit fly (or ‘Drosophila’) is often used as a model for genetics and developmental research.
- Its genome was published in 2000. Today, scientists can use this information to engineer and mutate fruit fly genes to understand how they work, and how that applies to humans.
- For example, the formation of several organs – including the eye – are controlled by equivalent genes in both humans and fruit flies.
- Drosophila fruit flies measure approximately 3 mm in length. Their larvae are small, white and glossy. The adults have brown and black stripes on their back and bright red eyes – although these characteristics are easily changed by manipulating their genes.

Limitations of using the fruit fly
- The anatomy of the brain and other major organs are very different between humans and Drosophila fruit flies.
- Although there are genes in the fruit fly that are equivalent to human genes, they don’t all act in the same way.
- We don’t have an easy measure of complex behaviours.
- Some drugs that work in the fruit fly don’t work or aren’t safe in humans.
- We don’t fully understand what could happen if genetically engineered animals entered the natural ecosystem.
- As with all animal research, there are ethical and moral questions to consider.
What other model organisms have had their genome sequenced?

Item added to your cart
Flying through history: nobel prizes for fruit fly research (part 1).
Drosophila melanogaster (D. mel) , a type of fruit fly, has been the subject of scientific curiosity for nearly a century. The extensive body of knowledge surrounding D. mel makes it the most well-known organism on the planet. Showing genetic similarities to humans, fruit flies have often been the model organism of choice for humanity’s genomic and developmental studies.
The scientific community has recognised the groundbreaking work utilising D. mel since the early 1900s. Over the course of a century, the Nobel Prize in Physiology or Medicine has been awarded six times to 11 scientists for their fruit fly findings:
- 1933: Thomas Hunt Morgan – The role played by chromosomes in heredity
- 1946: Hermann Joseph Muller – The production of mutations by means of X-ray irradiation
- 1995: Edward B. Lewis, Christiane Nüsslein-Volhard, Eric F. Wieschaus – The genetic control of early embryonic development
- 2004: Richard Axel and Linda Buck – Odour receptors and the organisation of the olfactory system (mainly rodent work)
- 2011: Jules A. Hoffmann – The activation of innate immunity
- 2017: Jeffrey C. Hall, Michael Rosbash, Michael W. Young – The discovery of molecular mechanisms controlling the circadian rhythm
Two months out from the 2022 Nobel Prize awardments, Future Fields is celebrating D. mel -based research that rocked our world for the better. Its EntoEngine™ platform would not exist today without the work done by these scientists.
Let’s first take a look at the Nobel Flies of the 1900s.
1933: A Fly Legacy Was Born
D. mel genetics studies arguably started with U.S. biologist Thomas Hunt Morgan, who discovered a mutation in fruit flies’ eye colour in 1910. Through a series of experiments that crossed white-eyed mutant flies with naturally red-eyed flies, Morgan demonstrated that the mutant gene for white eyes resided on the X chromosome. This meant that fly offspring could inherit new combinations of traits from their parents.
In the years following, Morgan used this basis to propose that genes are arranged on a chromosome like beads on a string. These beads contain genetic material and can be crossed over between a pair of chromosomes to create new genetic combinations. Morgan’s proposition transformed our understanding of genes from abstract ‘elements’ to tangible units, thus forming the foundation for the chromosome theory of heredity.
Morgan’s research was conducted in a small lab at Columbia University, which was later dubbed “The Fly Room.” Here, Morgan and his students pioneered the use of D. mel to study genetics, and his lab is often considered 'the birthplace of modern genetics research.' The Fly Room’s contributions towards our understanding of genetic mutations, sex-linked traits, and heredity earned Morgan the receipt of the Nobel Prize in Physiology or Medicine in 1933 . [1]
1946: Mulling Over Mutations
One of the trailblazers in Morgan’s fly lab was Hermann Joseph Muller, who demonstrated genetic changes through x-rays. From 1926 to 1927, Muller bred fruit flies exposed to varying levels of radiation in a series of three experiments. The resulting offspring had noticeable differences in genetic markers and birth rates, as well as body abnormalities. The prolonged exposure to x-rays triggered mutations (sudden changes in genetic codes), with increasing exposure leading to a greater number of mutations.
Muller’s findings showed scientists that environmental factors like radiation could disrupt normal genetic functions and heritable characteristics. This principle is still used today, as radiologists take precautions to shield human patients from harm. Muller’s fly experiments on the mutagenic effects of x-rays earned him the Nobel Prize in Physiology or Medicine in 1946 . [2]
1995: A Brave New Embryo
More than 50 years after Morgan’s and Muller’s experiments, two young molecular biologists on the other side of the world, Christiane Nüsslein-Volhard and Eric Wieschaus, joined forces to investigate how a freshly fertilised D. mel egg grew into a segmented embryo. Their experiments specifically looked into genetic malformations to determine the genes that control early embryonic development. Their analysis pinpointed 15 genes responsible for segmentation. This novel approach utilising fruit flies highlighted that genes could be systematically classified , and prompted the discovery of the same types of genes in higher order organisms, including humans.
At the same time as Nüsslein-Volhard and Wieschaus, California-based Edward B. Lewis was analysing the genetic basis for homeotic transformations, or how one organ transforms into another. Lewis was particularly interested in how the organ that controls balance in D. mel (the halteres) morphed into an extra pair of wings. He found that this occurence was due to inactivity of a certain gene along the D. mel embryo's body axis, which would originally form the halteres. Its inactivity caused a related gene to change the haltere gene into one that forms wings, creating the extra pair. Lewis’ work on homeotic gene interaction, segmentation, and expression was a crucial step in understanding developmental biology, as the D. mel homeotic analogues could be found in humans and other complex organisms.
Collectively, the three drosophilists opened a world of understanding for the early development of the human embryo, earning them the joint awardment of the Nobel Prize in Physiology or Medicine in 1995 . [3]
Nobel Flies of the 2000s coming soon. Follow our LinkedIn and Twitter for updates on our Nobel Flies series!
- Thomas H. Morgan – Biographical. NobelPrize.org. Nobel Prize Outreach AB 2022. Thu. 11 Aug 2022. https://www.nobelprize.org/prizes/medicine/1933/morgan/biographical/
- Hermann J. Muller – Biographical. NobelPrize.org. Nobel Prize Outreach AB 2022. Fri. 12 Aug 2022. https://www.nobelprize.org/prizes/medicine/1946/muller/biographical/
- Press release. NobelPrize.org. Nobel Prize Outreach AB 2022. Thu. 11 Aug 2022. https://www.nobelprize.org/prizes/medicine/1995/press-release/
More on the blog
Why Fruit Flies?
Future Fields - May 18, 2021
It’s in their genes: What makes fruit flies the ultimate model organism
Future Fields - Oct. 20, 2022
- Choosing a selection results in a full page refresh.

IMAGES
COMMENTS
4. Virgins fruit flies are physically distinctive from mature adults, making it easy to obtain virgin males and females for genetic crosses. 5. Flies have a short generation time (10-12 days) and do well at room temperature. 6. The care and culture of fruit flies requires little equipment, is low in cost and uses little space even for large ...
May 22, 2017 · In 1910, Thomas Hunt Morgan performed an experiment at Columbia University, in New York City, New York, that helped identify the role chromosomes play in heredity. That year, Morgan was breeding Drosophila, or fruit flies. After observing thousands of fruit fly offspring with red eyes, he obtained one that had white eyes.
Apr 5, 2024 · The Fruit Fly Lab-01 mission marked the first flight of a new research platform for long-duration experiments aboard the International Space Station. The Fruit Fly Lab system provided long-term housing for fruit flies, also known as Drosophila melanogaster, aboard the space station, under conditions of microgravity and simulated Earth gravity ...
Apr 2, 2018 · For more than 100 years, the fruit fly Drosophila melanogaster has played a starring role in biomedical research, revealing fundamental principles of genetics and development, illuminating human health and disease and earning scientists six Nobel prizes to date.
But before you launch into a genetics experiment, you might want to try this simpler experiment investigating fruit fly behavior. Ethology is the study of animal behavior. One type of behavior easily observed in fruit flies is called taxis (not to be confused with the vehicle; plural taxes ), which is the response of a living thing to an ...
The Drosophila melanogaster, or fruit fly, is a good genetic research subject because it can be bred cheaply and reproduces quickly. Morgan was not the first to use the fruit fly as a subject, but ...
Cai et al. 2010 / Journal of Visualized Experiments DOI: 10.3791/1748 . Limitations of using the fruit fly. The anatomy of the brain and other major organs are very different between humans and Drosophila fruit flies. Although there are genes in the fruit fly that are equivalent to human genes, they don’t all act in the same way.
Aug 11, 2022 · Let’s first take a look at the Nobel Flies of the 1900s. 1933: A Fly Legacy Was Born. D. mel genetics studies arguably started with U.S. biologist Thomas Hunt Morgan, who discovered a mutation in fruit flies’ eye colour in 1910. Through a series of experiments that crossed white-eyed mutant flies with naturally red-eyed flies, Morgan ...
the Fruit Fly Lab to enable fruit fly research aboard the space station. This hardware development project lever-ages the experience gained from prior flight experiments with fruit flies using a space shuttle-based system. Advanced capabilities of the new Fruit Fly Lab include providing environmental and behavioral monitoring
Jan 19, 2019 · Compare this with experiments on a few dozen chickens or mice… The fruit fly allowed geneticists to conduct experiments with an unprecedented statistical power. Today thousands of scientists around the world use Drosophila as a model organism for the same reasons Morgan used this pesky insect in his research over a century ago: