Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Oncology articles from across Nature Portfolio
Oncology is the branch of medicine that deals with the diagnosis and treatment of cancer. It includes medical oncology (the use of chemotherapy, hormone therapy, and other drugs to treat cancer), radiation oncology (the use of radiation therapy to treat cancer), and surgical oncology (the use of surgery and other procedures to treat cancer).
Immunosuppressive dura-derived macrophages in leptomeningeal metastasis
Leptomeningeal metastases of solid tumors are associated with poor prognosis, limited treatment options and unclear immunosurveillance mechanisms. Dura-derived immunosuppressive macrophages are now shown to migrate to the cerebrospinal fluid, limiting anti-tumor CD8 + T cell responses in models of leptomeningeal metastasis.
- Michael Kilian
- Francisco J. Quintana

Concurrently depleting regulatory T cells and activating cytotoxic T cells in tumours
An anti-4-1BB antibody fused to interleukin-15 via a peptide that is sensitive to tumour proteases concurrently depletes regulatory T cells and expands cytotoxic T cells in the tumour microenvironment. This fusion protein augments tumour inhibition without increasing systemic toxicity.
Perioperative nivolumab results in favourable long-term outcomes in patients with locally advanced resectable non-small-cell lung cancer
As one of the first studies testing perioperative anti-PD-(L)1 antibodies in resectable non-small-cell lung cancer (NSCLC), NADIM now confirms, in its final report, impressive 5-year clinical outcomes and that a pCR following neoadjuvant therapy translates into improved long-term survival. These data support the development of novel, personalized treatments for locally advanced resectable NSCLC.
- Tina Cascone
- William N. William Jr.
Related Subjects
- Surgical oncology
Latest Research and Reviews
Deep learning for endometrial cancer subtyping and predicting tumor mutational burden from histopathological slides
- Ching-Wei Wang
- Nabila Puspita Firdi
- Tai-Kuang Chao

Epidemiological landscape of tongue cancer in younger patients in a National Cancer Center in Brazil
- Ana Carolina da Silva Souto
- Beatriz Nascimento Monteiro da Silva
- Daniel Cohen Goldemberg
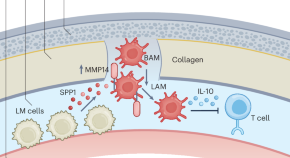
Dura immunity configures leptomeningeal metastasis immunosuppression for cerebrospinal fluid barrier invasion
Zhao et al. find that a macrophage subset migrates from the dura matter to reshape the cerebrospinal fluid microenvironment and promote metastasis and show that the secreted phosphoprotein 1–matrix metallopeptidase 14 axis can be targeted to inhibit this.
Breast cancer brain metastases genomic profiling identifies alterations targetable by immune-checkpoint and PARP inhibitors
- A. Giannoudis
- E. S. Sokol
- C. Palmieri
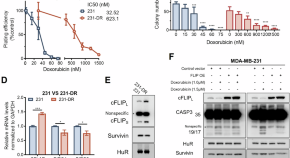
HuR inhibition overcomes cFLIP-mediated doxorubicin resistance in triple-negative breast cancer
- Lanjing Wei
- Sung Hae Kim
- Xiaoqing Wu
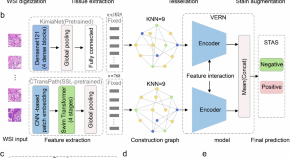
Feature-interactive Siamese graph encoder-based image analysis to predict STAS from histopathology images in lung cancer
- Liangrui Pan
- Qingchun Liang
- Shaoliang Peng
News and Comment
Pitfalls in interpreting calibration in comparative evaluations of risk models for precision lung cancer screening.
Lung cancer screening by low-dose computed tomography reduces lung cancer mortality, but reliable risk-based selection of participants is crucial to maximize benefits and minimize harms. Multiple risk models have been developed for this purpose, and their discrimination and calibration performance is commonly evaluated based on large-scale cohort studies. Using a recent comparative evaluation of 10 risk models as an example, we illustrate the merits, limitations and pitfalls of such evaluations.
- Hermann Brenner
- Clara Frick
- Megha Bhardwaj
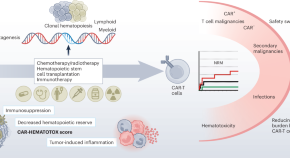
Navigating CAR-T cell therapy long-term complications
Chimeric antigen receptor (CAR)-T cells have revolutionized the treatment of hematological malignancies; however long-term safety concerns, associated with infections and secondary malignancies, have been reported. Here we discuss the clinical challenges of CAR-T cell therapy in light of this emerging benefit–risk controversy.
- Céline Grégoire
- J. Joseph Melenhorst
Commentary to: Cancer and treatment specific incidence rates of immune related adverse events induced by immune checkpoint inhibitors: a systematic review
- Alessandro Villa
- Bryan J. Schneider
- Douglas E. Peterson
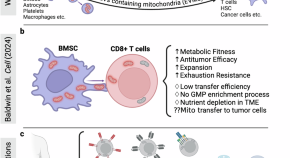
Bridge over troubled cells: bone marrow stromal cells transfer mitochondria to boost T cells
- Lars Fabian Prinz
- Roland Tillmann Ullrich
- Markus Martin Chmielewski
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Cancer Research
Driving discovery. Powering progress.
Nci research areas.
- Cancer Biology
- Causes of Cancer
- See All Research Areas
Cancer Research & Infrastructure at NCI
- NCI's Clinical Trials Programs
- NCI-Designated Cancer Centers
- Frederick National Laboratory for Cancer Research
- Intramural Research
- Extramural Research
- Cancer Research Workforce
Research Resources & NCI Initiatives
- Resources for Researchers
- The Cancer Moonshot℠
- Childhood Cancer Data Initiative
- Equity and Inclusion Program
- See All Key Initiatives
Research Advances

Explore the many ways you can participate in cancer research, including treatment and non-treatment studies.

NCI joins the cancer community in advancing the goals of the National Cancer Plan as part of its research programs.

NCI is advancing the use of AI applications in cancer research and care with the aim of using large volumes of data to create better outcomes for people with cancer.

Highlighted Scientific Opportunities
NCI highlights four areas of scientific opportunity for fiscal year 2026.

Oncology Research : Featuring Preclinical and Clinical Cancer Therapeutics
(Previously published as Cancer Communications)
Editors: Edward Chu, Kazuo Umezawa & Enrico Mini Volume 30, 2022
ISSN: 0965-0407; E-ISSN: 1555-3906 Open Access 4 numbers per volume
2021 IMPACT FACTOR: 5.574
CiteScore 2021: 11.8 View CiteScore for Oncology Research
This is an open access journal providing free, unrestricted online access to publisher content, open to all, with no access fees charged to the user and/or his/her institution.
Content can be read, downloaded, copied, distributed, printed, searched, linked to full text of articles and/or use for any other lawful purpose without obtaining permission from the publisher or author in accordance with the boai definition of open access..
https://www.budapestopenaccessinitiative.org/read
Go to previously published journal, Cancer Communications

Aims & Scope
Oncology Research is committed to publishing high-quality, innovative research that is focused on the entire range of preclinical, translational, and clinical cancer therapeutics. Specific areas of interest include preclinical and translational research in development of novel small molecules and targeted therapies; mechanisms of drug sensitivity; mechanisms of cellular drug resistance; biomarkers of response and/or resistance; novel experimental model systems and technologies relating to cancer therapeutics;pharmacogenetics and pharmacogenomics; personalized medicine; immunotherapy and clinical immunology; gene therapy; and radiobiology and novel approaches to radiation therapy either alone or in combination with chemotherapy. For studies that investigate the role of microRNAs and non-coding RNAs as regulators of cellular gene expression, it will be important for more in-depth mechanistic studies to be conducted that confirm their biological activity and their potential effect as mediators of chemosensitivity. As part of the preclinical cancer therapeutics focus, the journal also prioritizes preclinical studies that are focused on drug design, chemical biology, and drug screening. While the journal’s primary focus is on small molecules and protein drugs, other molecular entities may be considered. In addition, submissions that investigate the potential role of herbal/botanical medicines in preclinical and clinical cancer therapy are welcomed; however, it will be important to document that these medicines are of high quality, with confirmation of batch to batch consistency. In addition to original peer-reviewed articles, the journal also welcomes timely reviews and/or commentaries on topics that focus on preclinical, translational, and/or clinical cancer therapeutics
Co-Editors-in-Chief
American Continent: Edward Chu Albert Einstein College of Medicine, USA Email: [email protected]
Asia and Pacific Rim: Kazuo Umezawa Aichi Medical University School of Medicine, Japan Email: [email protected]
European Continent: Enrico Mini University of Florence, Italy Email: [email protected]
Associate Editor: Lois Malehorn EDITORIAL ADVISORY BOARD Alan C. Sartorelli ,† Founding Editor American Continent: Edward Chu, Co-Editor-in-Chief L. J. Appleman, University of Pittsburgh, USA N. Bahary, University of Pittsburgh, USA J. R. Bertino, Rutgers Cancer Institute of New Jersey, USA J. H. Beumer, Hillman Cancer Center, USA M. Boyiadzis, University of Pittsburgh, USA Y.-C. Cheng, Yale University School of Medicine, USA M. S. Copur, University of Nebraska, USA A. Krishnamurthy, University of Pittsburgh, USA Y. Li, Sorrento Therapeutics, USA G. D. Roodman, Indiana University, USA M. Rudek, Johns Hopkins University, USA J. C. Schmitz, University of Pittsburgh, USA N. Wei, University of Pittsburgh, USA L. Zhang, University of Pittsburgh, USA European Continent: Enrico Mini, Co-Editor-in-Chief A. H. Calvert, University of Newcastle upon Tyne, UK A. Di Paolo, University of Pisa, Italy D. Longley, Queen’s University, UK S. Nobili, University of Florence, Italy J. Robert, Université de Bordeaux, France J. H. M. Schellens, Netherlands Cancer Institute, the Netherlands P. Workman, CRC Center for Cancer Therapeutics, UK Asia and Pacific Rim: Kazuo Umezawa, Co-Editor-in-Chief A. Deguchi, Tokyo Women’s Medical University, Japan X. Ding, Nanjing Medical University, China S. Gantsev, Bashkirian State Medical University, Russia R. Horie, Kitasato University, Japan Y. Horiguchi, Tokyo Medical University, Japan M. Imoto, Keio University, Japan H. Kakeya, Kyoto University, Japan M. Kawatani, RIKEN, Japan E. Kikuchi, Keio University, Japan K. P. Kim, University of Ulsan College of Medicine, Korea T. W. Kim, University of Ulsan College of Medicine, Korea Y. Lin, Aichi Medical University, Japan J. Neuzil, Griffith University, Gold Coast Campus, Australia O. Ohno, Kogakuin University, Japan T. Ohsugi, Rakuno Gakuen University, Japan H. Osada, RIKEN, Japan M. Oya, Keio University, Japan M. Ozaki, Hokkaido University, Japan Y. Sasazawa, Juntendo University, Japan W. Seubwai, Khon Kaen University, Thailand K. Sidthipong, Mahidol University, Thailand S. Simizu, Keio University, Japan M. Takeiri, Ivy Cosmetics, Japan E. Tashiro, Keio University, Japan T. Ueno, Tsukuba University, Japan M. Yamamoto, Waseda University, Japan
Oncology Research uses CrossRef Similarity Check and is sustained by Portico Preservation Services .
INSTRUCTIONS TO CONTRIBUTORS
Open Access: Oncology Research is an open access journal and follows rules governed by open access publications. Accepted refereed articles published in the journal will be placed on the internet and will be publicly accessible, free of charge. In order to cover the costs of the journal, authors are expected to pay a publication fee. A $50.00 non refundable submission fee is required when your manuscript is sent out for review. You will also be asked to confirm that, if your manuscript is accepted for publication, you will pay the relatively inexpensive open access fee of $600.00 for less than 5 pages, or $1,000.00 for 5–12 pages and $50.00 for each additional page over 12 when billed at proof stage. The Open Access fee entitles the corresponding author to a free PDF file of the final version.
Color Options: Your article may contain figures that should be printed in color. There is a charge for figures appearing in color. The cost is $450.00 for figures appearing in color (this fee is for unlimited figures in one article).
Types of Contributions : The Journal publishes full-length papers and short communications, in English, describing the results of original experiments in basic and clinical cancer research. Commentaries, short research editorials of between 3,000 and 5,000 words in length (12–20 typewritten pages, double-spaced) are also published. These are editorial statements intended to stimulate thought on selected topics and should not be exhaustive reviews. They can be controversial and can focus on areas subject to much activity, or draw attention to relatively neglected fields in which there are both opportunities and the need for research. Authors may present personal views on the state of the subject on which they are reporting, and give their view as to where in the near or distant future the subject may be moving. Authors are encouraged to take issue with popular dogmas. Manuscripts are published in the shortest time possible commensurate with scientific quality.
Submission Requirements : Authors should submit the original manuscript electronically via email to [email protected] Send the text portion of the manuscript, including tables and figure legends, as an email attachment in Microsoft Word (IBM compatible) format. Send the figures as separate files (Microsoft Word, or as tiff or jpeg). Note that large graphic files, especially color, may need to be compressed (zipped) to send via email.
Include a cover letter, and insert “Oncology Research Submission” in the subject line of the email. The cover letter should contain the name, address, telephone, and fax number and electronic mail address of the author responsible for correspondence. Follow the General Manuscript Form guidelines below to prepare the manuscript, figures, and tables.
Each submitted manuscript is required to include at least three suggested reviewers, with affiliations, and email addresses. Authors should consider carefully their suggested reviewers. Suggested reviewers should not have a conflict of interest for the submitted manuscript, nor have substantial ties to the authors of the manuscript. Email addresses must be from the suggested reviewer’s institutional affiliation, not a non-specific, generic email address. Suggested reviewers should have expertise in the subject matter of the submitted manuscript. Note that the Editors-in-Chief retain the sole right to decide whether or not the suggested reviewers are used.
Manuscripts are accepted for consideration with the understanding that they have not been published elsewhere except in abstract form and are not concurrently under review elsewhere. Material accepted for publication will not be released publicly prior to its appearance in the journal. Authors are notified by the appropriate editorial office if the manuscript is accepted for publication.
General Manuscript Form: Manuscripts should be typed in English, double spaced throughout with at least 3-cm margins. Please consult the most recent issue of the journal for style and format. Number all pages consecutively, beginning with the title page. Use metric units of measure; other units may be given in parentheses. Typically, only three levels of headings are recognized. The manuscript should be organized as follows.
Title Pag e: The title should be brief and specific. The title page should contain in the following order: title, name(s) and affiliation(s) of author(s) including department institution, city, state, and country, and a suggested short title for the running head of not more than 50 characters and spaces. Also indicate the author to whom correspondence should be addressed and provide complete mailing address, telephone and fax numbers (optional), and e-mail address.
Abstract/Key Words: An abstract of 300 words or less should begin on page 2. It should contain a concise summary of the results, conclusions, and other significant points. For the purpose of subject indexing, provide four to six key words immediately following the abstract.
Text: Arrange the text with main headings of Introduction, Materials and Methods, Results, Discussion, Acknowledgments (and source of funding), References, Tables, Figure legends (together, and separate from the figures), and Figures (or as separate files). Use generic names of drugs. Give name, city and state, and country of the manufacturer of any chemicals, equipment, or software mentioned in the text. Define all nonstandard abbreviations the first time they appear in the text.
References: Literature cited should be prepared according to the Council of Science Editors format (citation-sequence system). This format is conveniently in Endnote and the output style is available at the following site: http://endnote.com/downloads/style/cse-style-manual-7th-ed-citation-sequence. Some examples are provided below. References in the text should be cited by superscript number separated by a comma and listed in numerical order as they appear in the text (double spaced) on a separate page at the end of the manuscript. Journal citations in the reference list should contain the following: (a) reference number (note NOT superscript); (b) surnames and initials of all authors (surnames precede initials); (c) title of article; (d) journal title abbreviated as listed in ISSN.org; (e) year; volume, inclusive pages . See the examples shown and refer to Council of Science Editors format for more examples.
Journal Article: 1. Roth CG, Gillespie-Twardy A, Marks S. Agha M, Raptis A, Hou JZ, Farah R, Lin Y, Qian Y, Pantanowitz L, Boyiadzis M. Flow cytometric evaluation of double/triple hit lymphoma. Oncol Res. 2016;23(3):137-46. Book: 1. Weinberg RA. The biology of cancer, 2nd ed. New York (NY): Garland Science; 2014. Book Article/Chapter: 1. Hasskarl J. Sorafenib: Targeting multiple tyrosine kinases in cancer. In: Martens UM, editor. Small molecules in oncology, 2nd ed. Berlin, Germany: Springer-Verlag; 2014. p. 145-164. Internet Source: 1. Cancer of the Colon and Rectum – SEER Stat Fact Sheets. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2011). Rockville (MD): National Cancer Institute; 2014 [accessed 2014 June 30]. http://seer.cancer.gov/statfacts/html/colorect.html
An example of an in-text citation is shown below.
Ovarian cancer is the third most common gynecological malignancy worldwide 1,2 .
To cite multiple sources, all numbers associated with the reference being cited should be superscript, separated by a comma, with no spaces between them.
Supplementary Material: Please note that the journal does not host supplementary material. If you wish to include supplementary material, then you will need to provide a link to a permanent hosting site of this material within the manuscript.
Tables: Tables should be numbered and cited sequentially in the text. Prepare each table as a separate page at the end of the manuscript text, after the references. Avoid very wide or long tables that would not fit a printed page. Each table should have a title, and each column in the table should have a brief heading. Define all abbreviations in the table footnote at the bottom of the table.
Figures: Figures should be numbered and cited sequentially in the text. Prepare figures to provide high quality suitable for reproduction. Avoid light lettering and shading that will not reproduce well. Figure dimensions and scaling should be suitable for reduction (if necessary) to fit column or page size. Care must be taken that letters and other symbols do not become so small that they are illegible when the figure is reduced. Complex formulas should be prepared as illustrations. After acceptance final figures should be provided in high resolution. Simple black and white figures (e.g., line graphs, bar graphs, etc.) should be 1200 dpi. Halftone and color figures (or combo figures) should be 600 dpi. Final figure files should be submitted as tiff, jpg, or eps format. Do not include the figure number as part of the figure file (e.g., do not label Figure 1, etc., as part of the figure). Do not provide color in a figure file unless the figure will be printed in color (note there is a cost for printing figures in color). ( Do not embed figures within the manuscript text. Prepare as separate files or at the end of the manuscript, after tables and figure legends . ) There is a cost to reproduce figures in color. The author is required to bear the costs for the publication of color figures (costs and color authorization form will be provided at proof stage).
Figure Legends: List all figure legends sequentially on one or more pages at the end of the manuscript text, after the references, and identify all symbols used in the figures. The figure legend should be as clear as possible and should fully describe the contents of the figure. ( Do not include the figure legend as part of the figure . ) If the figure is from a previously published article, indicate that permission has been obtained from the original publisher.
Use of Animal- and Human-derived tissue: Please confirm within the text that the appropriate ethical and/or regulatory body approved the use of animal- or human-derived tissue (as well as informed consent for humans). With human-derived data, articles are published on the understanding that appropriate measures to protect the privacy of the individuals were undertaken.
Permissions: If data from any other source is used in tables or figures it is the responsibility of the author(s) to obtain permission to reproduce such material. Provide proof that permission has been granted from the original publisher and indicate the source.
Page Proofs/Offprints: All material accepted for publication is subject to copyediting. Authors will receive page proofs of articles before publication, along with a Contributor’s Publishing Agreement , Open Access authorization form (with the final cost based on number of printed pages) and Color Figure authorization form (if there are potential color figures), which will need to be completed and returned before the article can be processed for publication. Only minor corrections are allowed at proof stage. Author can also request an offprint order form for ordering offprints or additional journal copies.
Copyright: Publications are copyrighted for the protection of authors and the publisher. A Transfer of Copyright Agreement will be sent to the corresponding author whose manuscript is accepted for publication. The form must be completed and returned with the final manuscript files(s).
Online Fast Track Publication : Accepted manuscripts will be loaded to Fast Track with DOI links online. Fast Track is an early e-pub system whereby subscribers to the journal can start reading and citing the articles prior to their inclusion in a journal issue. Please note that articles published in Fast Track are not the final print publication with proofs. Once the accepted manuscript is ready to publish in an issue of the journal, the corresponding author will receive a proof from our Production Department for approval. Once approved and published, the Fast Track version of the manuscript is deleted and replaced with the final published article. Online Fast Track publication ensures that the accepted manuscripts can be read and cited as quickly as possible.
Oncology Research (OR) Peer Review Policy
Peer review is the evaluation of scientific, academic, or professional work by others working in the same field to ensure that only good scientific research is published.
In order to maintain these standards, Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics ( OR) utilizes a single blind review process whereby the identity of the reviewers is not known to the authors but the authors are shown on the article being reviewed.
The peer review process for OR is laid out below:
A submitted article is forwarded to one of the Co-Editors (CE) based on the article’s country of origin. The CE determines if the article is within the scope of OR and whether it meets the basic standards of research.
If it is determined that the article should be forwarded to reviewers, the CE provides the Associate Editor (AE) with the names and contact information of at least two suggested reviewers for detailed peer review. The reviewers are always experts in their field and could be part of the OR editorial board. All reviewers would lack any conflict with the authors and are reviewers in good standing based on previous track record and history. Authors may not suggest reviewers; however, they are allowed to suggest reviewers to be avoided due to a potential conflict of interest.
Comments from the reviewers (minimum 2 reviewers) are expected in 2 weeks or less and are delivered to the AE. After the minimum is met, reviews are forwarded to the CE assigned to the submission. The CE then assesses the merit of the manuscript based on these comments as well as on their own assessment of the article. Special attention is given to declaration of conflict of interest if any.
If relevant, statements on use of appropriate animal protocol approved by institutional regulatory boards and inclusion of appropriate IRB approvals in cases of human studies are verified. Likewise, appropriate comments on use of appropriate statistical tests are ensured.
The CE provides the AE with their determination and authors receive detailed comments along with the final decision of: accept, accept with minor revision, accept with major revision, or rejection. The comments to authors are blinded.
As a reviewer for OR you would have the benefit of reading and evaluating current research in your area of expertise at its early stage, thereby contributing to the integrity of scientific exploration. If you are interested in becoming a reviewer for OR, please contact the Editor-in-Chief Edward Chu , University of Pittsburgh, USA at [email protected]
Ethics Statement
The publishers and editorial board of Oncology Research have adopted the publication ethics and malpractice statements of the Committee on Publication Ethics (COPE) https://publicationethics.org/core-practices . These guidelines highlight what is expected of authors and what they can expect from the reviewers and editorial board in return. They also provide details of how problems will be handled. Briefly :
Author Responsibilities: Authors listed on a manuscript must have made a significant contribution to the study and/or writing of the manuscript. During revisions, authors cannot be removed without their permission and that of the other authors. All authors must also agree to the addition of new authors. It is the responsibility of the corresponding author to ensure that this occurs.
Financial support and conflicts of interest for all authors must be declared. Further information on this can be obtained from the International Committee for Medical Journal Editors (http://www.icmje.org/).
The reported research must be novel and authentic and the authors should confirm that the same data has not been and is not going to be submitted to another journal (unless already rejected). Statements made in the introduction and discussion should be supported by appropriate references and sufficient experimental detail should be provided to allow for repetition of the study by another group. Plagiarism of the text/data will not be tolerated and could result in retraction of an accepted article. Any text or figures reproduced for another source require the permission of the original copyright holders (normally the publishers).
Any manipulation of figures should be equally applied and described in the text including pseudo-coloring and must not change the meaning of the figure.
When humans, animals or tissue derived from them have been used, then mention of the appropriate ethical approval must be included in the manuscript.
Author must sign a Transfer of Copyright form giving rights to the Publisher, at proof stage.
Reviewer Responsibilities: Reviewers are expected to not possess any conflicts of interest with the authors and research. They should review the science objectively and provide recommendations for improvements where necessary. When aware of relevant published work not being cited, the reviewers should recommend inclusion of these references. If the reviewer feels that they would be unable to repeat the study as described, then additional methodological details should be requested. Any unpublished information read by a reviewer should be treated as confidential.
Editorial Responsibilities: The section editors are expected to select an appropriate number of reviewers for the manuscript so that they can make an informed decision about whether to reject/accept a manuscript. Their decision must be based only on the paper’s importance, originality and clarity and whether it is suitable for the journal. They must not have a conflict of interest with the authors or work described. The anonymity of the reviewers must be maintained.
Should problems come to light after acceptance then the editors agree to promote the publication of corrections and/or retractions as deemed necessary.
NIH Public Access Policy : Cognizant Communication Corporation uploads published manuscripts on the authors’ behalf to PubMed Central so authors may remain in compliance with the NIH Public Access Policy.
Publishing Responsibilities: The publishers agree to ensure that to the best of their abilities, the information that they publish is genuine and ethically sound. If publishing ethics issues come to light, not limited to accusations of fraudulent data or plagiarism, during or after the publication process, they will be investigated by the editorial board including contact with the authors’ institutions if necessary, so that a decision on the appropriate corrections, clarifications or retractions can be made. The publishers agree to publish this as necessary so as to maintain the integrity of the academic record.
View All Abstracts
Access Current Articles (Volume 29, Number 1)
Volume 29, Number 1
Original Contributions
MYCN Directly Targets NeuroD1 to Promote Cellular Proliferation in Neuroblastoma – 1 https://doi.org/10.3727/096504021X16401852341873
Fangjin Lu,* Bin Mu,† Ge Jin,* Lin Zhu,‡ and Ping Mu§
*Department of Pharmacology, Shenyang Medical College, Shenyang, P.R. China †Shanghai Zhaohui Pharmaceutical Co. Ltd., Shanghai, P.R. China ‡Department of Biochemistry and Molecular Biology, Shenyang Medical College, Shenyang, P.R. China §Department of Physiology, Shenyang Medical College, Shenyang, P.R. China
NeuroD1 is a neuronal differentiation factor that contains a basic helix–loop–helix (bHLH) motif. Recently, NeuroD1 was found to be associated with tumorigenesis in neuroblastoma (NB) and is known to promote cell proliferation and migration in these cells. Here we found that MYCN regulates the expression of NeuroD1 in NB cells and that the downregulation of MYCN using short hairpin RNAs (shRNA) results in the inhibition of cellular proliferation in NB cells. Moreover, the phenotype induced by MYCN shRNA was rescued by the exogenous expression of NeuroD1. Chromatin immunoprecipitation (ChIP) assay showed that MYCN directly binds to the E-box element in the NeuroD1 promoter region. In addition, our evaluation of two clinical databases showed that there was a positive correlation between the expression of MYCN and NeuroD1 in NB patients, which supports our in vitro data. In conclusion, this study demonstrates that MYCN-regulated NeuroD1 expression is one of the important mechanisms underlying enhanced cellular proliferation induced by the increase in MYCN expression in NB, and our results provide an important therapeutic target for NB in the future.
Key words: Neuroblastoma; Cellular proliferation; NeuroD1; MYCN
STAT3 Polymorphism Associates With mTOR Inhibitor-Induced Interstitial Lung Disease in Patients With Renal Cell Carcinoma – 11 https://doi.org/10.3727/096504022X16418911579334
Kazuhiro Yamamoto,* Takeshi Ioroi,* Kazuaki Shinomiya,† Ayaka Yoshida,* Kenichi Harada,‡ Masato Fujisawa,‡ Tomohiro Omura,* Yasuaki Ikemi,§ Shunsaku Nakagawa,§ Atsushi Yonezawa,§ Osamu Ogawa,¶ Kazuo Matsubara,§ Takuya Iwamoto,# Kohei Nishikawa,** Sayaka Hayashi,†† Daichi Tohara,†† Yoji Murakami,‡‡ Takanobu Motoshima,‡‡ Hirofumi Jono,†† and Ikuko Yano*§
*Department of Pharmacy, Kobe University Hospital, Kobe, Japan †Department of Pharmaceutical Care and Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Tokushima, Japan ‡Division of Urology, Kobe University Graduate School of Medicine, Kobe, Japan §Department of Clinical Pharmacology and Therapeutics, Kyoto University Hospital, Kyoto, Japan ¶Department of Urology, Graduate School of Medicine, Kyoto University, Kyoto, Japan #Department of Pharmacy, Mie University Hospital, Mie, Japan **Department of Nephro-Urologic Surgery and Andrology, Mie University Graduate School of Medicine, Mie, Japan ††Department of Pharmacy, Kumamoto University Hospital, Kumamoto, Japan ‡‡Department of Urology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan
We evaluated the association of signal transducer and activator of transcription 3 ( STAT3 ) polymorphisms with the incidence of mammalian target of rapamycin (mTOR) inhibitor-induced interstitial lung disease (ILD) in patients with renal cell carcinoma (RCC). We also used lung-derived cell lines to investigate the mechanisms of this association. Japanese patients with metastatic RCC who were treated with mTOR inhibitors were genotyped for the STAT3 polymorphism, rs4796793 (−1697C/G). We evaluated the association of the STAT3 genotype with the incidence of ILD and therapeutic outcome. In the 57 patients included in the primary analysis, the ILD rate within 140 days was significantly higher in patients with the GG genotype compared with those with other genotypes (77.8% vs. 23.1%, odds ratio = 11.67, 95% confidential interval = 3.06–44.46). There were no significant differences in progression-free survival or time-to-treatment failure between the patients with the GG genotype and those with other genotypes. An in vitro study demonstrated that some lung-derived cell lines carrying the GG genotype exhibited an increase in the expression of mesenchymal markers, such as fibronectin, N-cadherin, and vimentin, and decreases in E-cadherin, which is an epithelial marker associated with exposure to everolimus, although STAT3 expression and activity were not related to the genotype. In conclusion, the GG genotype of the STAT3 rs4796793 polymorphism increases the risk of mTOR inhibitor-induced ILD, supporting its use as a predictive marker for RCC.
Key words: mTOR inhibitor; Interstitial lung disease; STAT3; Polymorphism; Epithelial–mesenchymal transition (EMT)
Real-World Data: Fruquintinib in Treating Metastatic Colorectal Cancer – 25 https://doi.org/10.3727/096504022X16427607626672
Shuai Liu,*† Lu Lu,*† Feng Pan,‡ Chunsheng Yang,*† Jing Liang,§ Jinfeng Liu,¶ Jian Wang,# Rong Shen,** Fu-Ze Xin,†† and Nan Zhang*†
*Department of Breast Disease Diagnosis and Treatment Center, Central Hospital Affiliated to Shandong First Medical University, Jinan, P.R. China †Department of Breast Disease Diagnosis and Treatment Center, Jinan Central Hospital, Cheeloo College of Medicine, Shandong University, Jinan, P.R. China ‡Ethics Committee Office, Jinan Central Hospital, Cheeloo College of Medicine, Shandong University, Jinan, P.R. China §Department of Oncology, Shandong Provincial Qianfoshan Hospital, Jinan, P.R. China ¶Department of Oncology, Rizhao Hospital of Traditional Chinese Medicine, Rizhao, P.R. China #Department of Medical Oncology, Qilu Hospital of Shandong University, Jinan, P.R. China **Department of Chemotherapy, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, P.R. China ††Department of Gastrointestinal Surgery, Liao Cheng People’s Hospital, Liaocheng, P.R. China
Fruquintinib, also called HMPL-013, was first discovered by Hutchison Whampoa Pharmaceuticals Co. Ltd., Shanghai, China, and it is an oral vascular endothelial growth factor receptor (VEGFR) inhibitor. In clinical trials, fruquintinib has demonstrated a survival benefit in metastatic colorectal cancer (mCRC) patients. The purpose of this study was to retrospectively evaluate the efficacy and toxicity of fruquintinib in real-world patients. We collected data from patients with mCRC treated with oral fruquintinib from 2018 to 2020 in six different institutions. Patients with mCRC initially received 5 mg of oral fruquintinib daily for 3 weeks. Progression-free survival (PFS) was evaluated using the Kaplan–Meier method. The efficacy and safety of fruquintinib were also assessed. Seventy-five patients were involved in our study, and 29.3% of patients achieved stable disease (SD). Median PFS was 5.4 months (95% CI: 4.841–5.959). The treatment-emergent adverse events (TEAEs) with fruquintinib were acceptable with grade 3 TEAEs of 6%. The grade 3 TEAEs were hand–foot skin reaction (HFSR), fatigue, and stomatitis. The ECOG performance status was associated with PFS. In this real-world study, the clinical activity of fruquintinib was consistent with what has been reported in previous clinical trials. The level of safety was acceptable, and the side effects were manageable.
Key words: Metastatic colorectal cancer (mCRC); Fruquintinib; Efficacy; Safety
Lactate Maintains BCR/Abl Expression and Signaling in Chronic Myeloid Leukemia Cells Under Nutrient Restriction – 33 https://doi.org/10.3727/096504022X16442289212164
Angela Silvano,*† Giulio Menegazzi,* Silvia Peppicelli,* Caterina Mancini,* Alessio Biagioni,* Alessandro Tubita,* Ignazia Tusa,* Jessica Ruzzolini,* Matteo Lulli,* Elisabetta Rovida,* and Persio Dello Sbarba*
*Department of Experimental and Clinical Biomedical Sciences “Mario Serio,” Careggi Hospital, Universita degli Studi di Firenze, Florence, Italy †Department of Health Sciences, Division of Obstetrics and Gynecology, Careggi Hospital, Universita degli Studi di Firenze, Florence, Italy
This study was directed to deepen the effects of nutrient shortage on BCR/Ablprotein expression and signaling in chronic myeloid leukemia (CML) cells. The backbone of the study was cell culture in medium lacking glucose, the consumption of which we had previously shown to drive BCR/Ablprotein suppression, and glutamine, the other main nutrient besides glucose. In this context, we focused on the role of lactate, the main by-product of glucose metabolism under conditions of rapid cell growth, in particular as a modulator of the maintenance of CML stem/progenitor cell potential, a crucial determinant of disease course and relapse of disease. The results obtained indicated that lactate is a powerful surrogate of glucose to prevent the suppression of BCR/Abl signaling and is therefore capable to maintain BCR/Abl-dependent CML stem/progenitor cell potential. A number of metabolism-related functional and phenotypical features of CML cells were also determined. Among these, we focused on the effect of lactate on oxygen consumption rate, the dependence of this effect on the cell surface lactate carrier MCT-1, and the relationship of the lactate effect to pyruvate and to the activity of mitochondrial pyruvate carrier.
Key words: Chronic myeloid leukemia (CML); Nutrient shortage; Lactate; Stem cell potential; Acidosis; MCT-1
Comparison of UGT1A1 Polymorphism as Guidance of Irinotecan Dose Escalation in RAS Wild-Type Metastatic Colorectal Cancer Patients Treated With Cetuximab or Bevacizumab Plus FOLFIRI as the First-Line Therapy – 47 https://doi.org/10.3727/096504022X16451187313084
Hsiang-Lin Tsai,*† Yen-Cheng Chen,*‡ Tzu-Chieh Yin,*§¶ Wei-Chih Su,*‡ Po-Jung Chen,* Tsung-Kun Chang,*† Ching-Chun Li,* Ching-Wen Huang,*† and Jaw-Yuan Wang*†‡#**††‡‡
*Division of Colorectal Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan †Department of Surgery, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan ‡Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan §Division of General and Digestive Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan ¶Department of Surgery, Kaohsiung Municipal Tatung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan #Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan **Center for Cancer Research, Kaohsiung Medical University, Kaohsiung, Taiwan ††Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University, Kaohsiung, Taiwan ‡‡Pingtung Hospital, Ministry of Health and Welfare, Pingtung, Taiwan
Uridine diphosphate glucuronosyltransferase 1A1 ( UGT1A1 ) polymorphism plays a crucial role in the increased susceptibility and toxicity of patients to irinotecan. This retrospective, observational study compared the clinical outcomes and adverse events (AEs) in RAS wild-type metastatic colorectal cancer (mCRC) patients treated with cetuximab or bevacizumab plus FOLFIRI with UGT1A1 genotyping and irinotecan dose escalation as the first-line therapy. In total, 173 patients with mCRC with RAS wild-type were enrolled. Among them, 98 patients were treated with cetuximab, whereas 75 patients were treated with bevacizumab. All patients received irinotecan dose escalation based on UGT1A1 genotyping. We compared the progression-free survival (PFS), overall survival (OS), objective response rates (ORRs), disease control rates (DCRs), metastatectomy, and severe adverse events (SAEs) between the two groups. The clinical effects of primary tumor sidedness and target therapy crossover were further analyzed. Over a median follow-up of 23.0 months [interquartile range (IQR), 15.0–32.5 months], no significant differences were observed between the cetuximab and bevacizumab groups in PFS [18.0 months vs. 14.0 months; 95% confidence interval (CI), 0.517–1.027; hazard ratio (HR), 0.729; p = 0.071], OS (40.0 months vs. 30.0 months; 95% CI, 0.410–1.008; HR, 0.643; p = 0.054), ORR (65.3% vs. 62.7%; p = 0.720), DCR (92.8% vs. 86.7%; p = 0.175), metastatectomy (36.7% vs. 29.3%; p = 0.307), and SAEs ( p = 0.685). Regardless of primary tumor sidedness and target therapy crossover, no significant differences were noted in efficacy and safety between the two groups (all p > 0.05). Our results revealed that patients with wild-type RAS mCRC, regardless of biologics, with UGT1A1 genotyping can tolerate escalated doses of irinotecan and potentially achieve a more favorable clinical outcome without significantly increased toxicity.
Key words: UGT1A1 polymorphism; Metastatic colorectal cancer (mCRC); Irinotecan dose escalation; Biologics; Efficacy; Safety
Research Progress in Immunotherapy of NSCLC With EGFR-Sensitive Mutations – 63 https://doi.org/10.3727/096504022X16462176651719
Yudie Yang,* Xia Zhang,† Yajie Gao,* Yan Dong,* Di Wang,* Yanping Huang,* Tianhao Qu,* Buqun Fan,* Qizheng Li,* Chunxia Zhang,* Xiaonan Cui,* and Bin Zhang*
*Department of Oncology, The First Affiliated Hospital of Dalian Medical University, Dalian, P.R. China †Department of Oncology, Dalian Fifth People’s Hospital, Dalian, P.R. China
Lung cancer is a malignant tumor with high incidence and mortality across the world. The use of immune checkpoint inhibitors for lung cancer has improved the prognosis of some lung cancer patients to a greater extent and provided a new direction for the clinical treatment of lung cancer. Immunotherapy still has limitations in terms of its appropriate population and adverse reactions. Particularly for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutation, there has been no major breakthrough in current immunotherapy. Whether immunotherapy can bring new benefits after drug resistance is induced by tyrosine kinase inhibitor-targeted therapy and whether the combination of immunotherapy with other treatments can improve the prognosis remain to be studied in depth. In this article, we provide a detailed review of the relevant characteristics of the tumor microenvironment of NSCLC with EGFR mutation and the current research on immunotherapy for NSCLC with EGFR mutation.
Key words: Non-small cell lung cancer (NSCLC); Immunotherapy; Epidermal growth factor receptor (EGFR)-sensitive mutations
Retractions
Retraction notice to “Long Noncoding RNA LINC01133 Functions as an miR-422a Sponge to Aggravate the Tumorigenesis of Human Osteosarcoma” [Oncology Research 26(3) (2018) 335–343] – 75 https://doi.org/10.3727/096504022X16420719767471
Hai-Feng Zeng,* Hai-Yan Qiu,† and Fa-Bo Feng‡
*Department of Plastic Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, P.R. China †Department of Endocrinology, Hangzhou First People’s Hospital, Nanjing Medical University, Nanjing, P.R. China ‡Department of Orthopedics, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, P.R. China
Retraction notice to “Targeted Silencing of Kim-1 Inhibits the Growth of Clear Cell Renal Cell Carcinoma Cell Line 786-0 In Vitro and In Vivo” [Oncology Research 26(7) (2018) 997–1003] – 77 https://doi.org/10.3727/096504022X16425915715943
Jianping Xu, Liguo Sun, Wei Sun, Jianhai Tian, and Huaiyuan Guo
Department of Urology, Tumor Hospital of Linyi City, Lanshan, Linyi, Shandong, P.R. China
Retraction notice to “Downregulation of MicroRNA-147 Inhibits Cell Proliferation and Increases the Chemosensitivity of Gastric Cancer Cells to 5-Fluorouracil by Directly Targeting PTEN” [Oncology Research 26(6) (2018) 901–911] – 79 https://doi.org/10.3727/096504022X16491544361323
Jianjun Shen,* Weina Niu,† Hongbo Zhang,* Ma Jun,* and Hongyan Zhang*
*Department of Radiation Oncology, Anhui Provincial Hospital, Hefei, Anhui, P.R. China †Department of Oncology, Anhui Cancer Hospital, Hefei, Anhui, P.R. China
Full text articles available: CLICK HERE
Back issues of this journal are available online. Order Here
Oncology Research is indexed in:
Web of Science Coverage: SCIENCE CITATION INDEX EXPANDED BIOLOGICAL ABSTRACTS BIOSIS FULL COVERAGE SHARED BIOSIS PREVIEWS CC/LIFE SCIENCES CURRENT CONTENTS PROCESSING CODE DERWENT DRUG FILE ESSENTIAL SCIENCE INDICATORS JOURNAL CITATION REPORTS SCIENCE PROUS RESEARCH ALERT
CABI CABS/ELSEVIER BIOBASE CHEMICAL ABSTRACTING SERVICES EBSCO DISCOVERY SERVICES-EDS GOOGLE ANALYTICS MEDLINE PRIMO CENTRAL PROQUEST SCOPUS SIIC/SOCIEDAD LIBEROAMERICANA DE THOMSON REUTERS WORLDCAT DISCOVERY SERVICES
This journal uses CrossRef Similarity Check and is sustained by Portico Preservation Services.
Publishing Information
Before publication Cognizant, LLC ./ Cognizant Communication Corporation (collectively “CCC”) requires the author(s) as the rights holder, to sign a Journal Contributor’s Publishing Agreement. Manuscripts published in Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics fall under Creative Commons License CC-BY-NC-ND 4.0 ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ). For more information please visit CCC’s Reusing Open Access Content Policy .
It is a condition of publication that manuscripts submitted to this Journal have not been published and will not be simultaneously submitted or published elsewhere.
Although every effort is made by the publisher and editorial board to see that no inaccurate or misleading data, opinion, or statement appears in this journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the sole responsibility of the contributor or advertiser concerned. Accordingly, the publisher, editors, editorial board, and their respective employees, officers, and agents accept no responsibility or liability whatsoever for the consequences of any such inaccurate or misleading data, opinions, or statements.
The authors have made every effort to ensure the accuracy of the information herein, particularly with regard to drug selection and dose. However, appropriate information sources should be consulted, especially for new or unfamiliar drugs or procedures. It is the responsibility of every practitioner to evaluate the appropriateness of a particular opinion in the context of actual clinical situations and with due consideration to new developments.
CREATIVE COMMONS LICENSE Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics is Open Access under the terms of the Creative Commons CC BY-NC-ND license. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ )
Copyright © 2021 Cognizant, LLC
Printed in the USA
On behalf of my co-editors, Drs. Mini and Umezawa, we would like to respond to the negative comments that have been recently voiced on social media regarding published material in Oncology Research . As co-editors we can assure the public that we are deeply committed to ensuring a fair and appropriate review process for the journal. When a manuscript is sent out for review, we work hard to identify appropriate reviewers with special expertise in the research area that is covered in the submitted manuscript, and admittedly, we rely heavily on the reviews that are submitted by our expert reviewers. As editors, we then go over the reviews submitted and then make a decision for the manuscript. In the event that a manuscript needs to be revised, we ensure that all of the major reviewers’ comments are addressed and incorporated in the revised manuscript and, in most cases, will return the revised manuscript to the original group of reviewers to make sure that they are in agreement with publication. Given the recent issues surrounding the possibility of plagiarism, we have now instituted a similarity check process to ensure that the revised manuscript is high-quality, original research. However, the issue with proposing to have a similarity check process is “how does one actually search and find similar blots in all of PubMed?” Many of the identified plagiarized images are from different institutions with different authors. While there has been talk of developing software to find duplicate images, we do not believe that this is yet ready for prime time. We may also get some questions or pushback as to how our journal will actually institute such a process.
In all our collective years of reviewing manuscripts and editing this journal as well as others, we have never faced a situation where so many issues relating to published manuscripts have been identified. We firmly believe in a review process that is transparent, fair, and rigorous, and we believe in publishing high-quality scientific research. We also firmly believe that authors need to take ownership over this process and that they need to be honest and transparent when they submit a manuscript to Oncology Research or any other journal. Unfortunately, with modern technology, it has become even easier for manipulation of scientific data to occur. While we firmly believe in the integrity of the review process, it is clear that there are some authors willing to skirt the truth and present falsified data. As such, we need to be even more rigorous and vigilant in our efforts to review manuscripts. At this time, we will take whatever corrective actions are necessary to ensure the integrity of our journal and our good names as editors. We have already requested that several of the manuscripts under question be retracted and/or for the authors to provide additional information that addresses concerns relating to their published work. We plan to move forward with stricter review and acceptance policies, will remind our reviewers to take an even more rigorous approach to their review of submissions, and will have all potentially accepted manuscripts go through a similarity check to rule out the possibility of plagiarism.
Working together as a team to resolve these various issues, we are highly confident that we can restore the integrity of the journal.
Respectfully,
The Co-Editors of Oncology Research Edward Chu, Enrico Mini, and Kazuo Umezawa
Updated as of December 2021
Number of submissions: 91 Number of reviews requested: 133 Number of reviews received: 42 Approval rate: 10% Average time between submission and publication: 8.6 months
Robert N. Miranda, Publisher/Chairman Lori H. Miranda, President/COO
P.O. Box 37 Putnam Valley, NY 10579 USA
Phone: (845) 603-6440 Fax: (845) 603-6442
[email protected] [email protected]
About Cognizant
Cognizant Policies
Publications
Active Journals
Previously Published Journals

Privacy Policy | Terms of Use | Contact Us | Help
Copyright ©2024 Cognizant LLC. All rights reserved Cognizant Communication Corporation | P.O. Box 37 | Putnam Valley, NY 10579 | U.S.A.

National Cancer Institute - Cancer.gov
Center for Cancer Research
Creating the cancer medicines of tomorrow through bold biomedical research
Latest News

Celebrating CCR Careers: Jay A. Berzofsky, M.D., Ph.D.

Empowering others to contribute to rare neuroendocrine tumor research

An observant patient opens new doors for cancer screening

Clinical Trials
Our highly trained physicians offer clinical trials for patients with cancer, HIV or immunodeficiency disorders. All trials take place at the NIH Clinical Center in Bethesda, MD, and through telehealth. Once you are enrolled in a clinical trial at the Clinical Center, medical care is free.

Centrally supported by long-term funding and a culture of complete intellectual freedom, CCR scientists are able to pursue the most important and challenging problems in cancer research.

Training Opportunities
Our innovative training programs educate and mentor the next generation of researchers and physician-scientists, from summer students to faculty members. We can help you launch your career in basic, clinical, or translational research.
What can we help you find?

IMAGES